当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Facile Synthesis of Chiral Arylamines, Alkylamines and Amides by Enantioselective NiH-Catalyzed Hydroamination
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2021-08-27 , DOI: 10.1002/anie.202109881 Lingpu Meng 1 , Jingjie Yang 1 , Mei Duan 1 , You Wang 1 , Shaolin Zhu 1
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2021-08-27 , DOI: 10.1002/anie.202109881 Lingpu Meng 1 , Jingjie Yang 1 , Mei Duan 1 , You Wang 1 , Shaolin Zhu 1
Affiliation
|
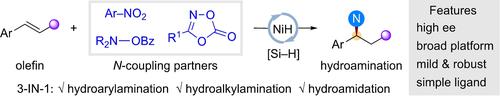
Regio- and enantioselective hydroarylamination, hydroalkylamination and hydroamidation of styrenes have been developed by NiH catalysis with a simple bioxazoline ligand under mild conditions. A wide range of enantioenriched benzylic arylamines, alkylamines and amides can be easily accessed by nitroarenes, hydroxylamines and dioxazolones, respectively as amination reagents. The chiral induction in these reactions is proposed to proceed through an enantiodifferentiating syn-hydronickellation step.
中文翻译:

对映选择性 NiH 催化加氢胺化反应简便合成手性芳胺、烷基胺和酰胺
苯乙烯的区域选择性和对映选择性加氢芳基化、加氢烷基胺化和加氢酰胺化已在温和条件下通过 NiH 催化与简单的生物恶唑啉配体开发。硝基芳烃、羟胺和二恶唑酮分别作为胺化试剂可以很容易地获得各种富含对映体的苄基芳胺、烷基胺和酰胺。这些反应中的手性诱导被提议通过对映体分化的顺式氢镍化步骤进行。
更新日期:2021-10-19
中文翻译:

对映选择性 NiH 催化加氢胺化反应简便合成手性芳胺、烷基胺和酰胺
苯乙烯的区域选择性和对映选择性加氢芳基化、加氢烷基胺化和加氢酰胺化已在温和条件下通过 NiH 催化与简单的生物恶唑啉配体开发。硝基芳烃、羟胺和二恶唑酮分别作为胺化试剂可以很容易地获得各种富含对映体的苄基芳胺、烷基胺和酰胺。这些反应中的手性诱导被提议通过对映体分化的顺式氢镍化步骤进行。


























 京公网安备 11010802027423号
京公网安备 11010802027423号