Synthesis ( IF 2.6 ) Pub Date : 2021-08-26 , DOI: 10.1055/s-0037-1610783 John J. Keating 1, 2, 3 , Ryan M. Alam 1, 2
|
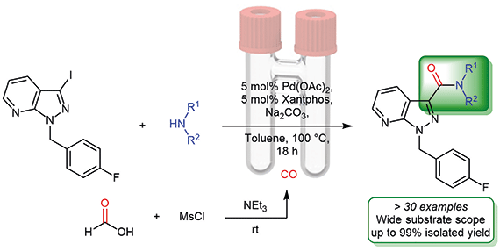
Pyrazolo[3,4-b]pyridine is a privileged scaffold found in many small drug molecules that possess a wide range of pharmacological properties. Efforts to further develop and exploit synthetic methodologies that permit the functionalization of this heterocyclic moiety warrant investigation. To this end, a series of novel 1,3-disubstituted pyrazolo[3,4-b]pyridine-3-carboxamide derivatives have been prepared by introducing the 3-carboxamide moiety using palladium-catalyzed aminocarbonylation methodology and employing CO gas generated ex situ using a two-chamber reactor (COware®). The functional group tolerance of this optimized aminocarbonylation protocol is highlighted through the synthesis of a range of diversely substituted C-3 carboxamide pyrazolo[3,4-b]pyridines in excellent yields of up to 99%.
中文翻译:

一种通过钯催化氨基羰基化制备 Pyrazolo[3,4-b]pyridine-3-carboxamides 的权宜之计
吡唑并[3,4- b ]吡啶是一种在许多具有广泛药理特性的小药物分子中发现的特殊支架。进一步开发和利用合成方法的努力,允许这种杂环部分的功能化值得研究。为此,通过使用钯催化的氨基羰基化方法引入 3-羧酰胺部分并使用异位产生的 CO 气体制备了一系列新型 1,3-二取代的吡唑并[3,4- b ]吡啶-3-羧酰胺衍生物。使用两室反应器 (COware ® )。通过合成一系列不同取代的 C-3 羧酰胺吡唑并 [3,4-b ]吡啶,产率高达 99%。



























 京公网安备 11010802027423号
京公网安备 11010802027423号