Tetrahedron ( IF 2.1 ) Pub Date : 2021-08-27 , DOI: 10.1016/j.tet.2021.132427 Xiao-bo Zhao 1 , Yi-meng Wang 1 , Hai-feng Yu 1 , Yuan-cheng Lv 1 , Si-ao Jiang 1 , Nan Wang 1
|
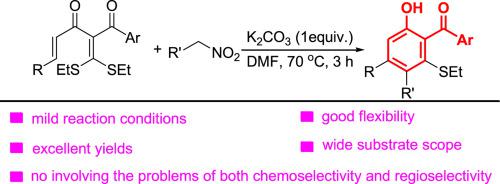
A new and efficient protocol for the synthesis of 2-hydroxybenzophenone derivatives through [5C+1C] annulation of α-alkenoyl-α-aroyl ketene dithioacetals and nitroalkanes had been developed. In the presence of 1 equivalent of K2CO3 in DMF at 70 °C, a range of α-alkenoyl-α-aroyl ketene dithioacetals smoothly react with nitroalkanes to provide various 2-hydroxy benzophenone derivatives in excellent yields. The protocol featured mild reaction conditions, no the formation of by-products, excellent yield, no involving the problems of both chemoselectivity and regioselectivity and wide substrate scope.
中文翻译:

通过α-烯酰基-α-芳酰基乙烯酮二硫缩醛和硝基烷烃的[5C + 1C]环化制备2-羟基二苯甲酮衍生物的新方法
开发了一种通过 α-烯酰基-α-芳酰基烯酮二硫缩醛和硝基烷烃的 [5C+1C] 环化合成 2-羟基二苯甲酮衍生物的新的有效方案。在70°C 下,在 DMF 中存在 1 当量 K 2 CO 3时,一系列 α-烯酰基-α-芳酰基乙烯酮二硫缩醛与硝基烷烃顺利反应,以优异的产率提供各种 2-羟基二苯甲酮衍生物。该方案反应条件温和,无副产物生成,收率高,不存在化学选择性和区域选择性并存的问题,底物范围广。



























 京公网安备 11010802027423号
京公网安备 11010802027423号