当前位置:
X-MOL 学术
›
ACS ES&T Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Investigation of Surfactant–Membrane Interaction Using Molecular Dynamics Simulation with Umbrella Sampling
ACS ES&T Engineering Pub Date : 2021-08-26 , DOI: 10.1021/acsestengg.1c00262 Yunqiao Ma 1, 2, 3 , Sadiye Velioğlu 4 , Thein An Trinh 1, 2 , Rong Wang 2, 5 , Jia Wei Chew 1, 2
ACS ES&T Engineering Pub Date : 2021-08-26 , DOI: 10.1021/acsestengg.1c00262 Yunqiao Ma 1, 2, 3 , Sadiye Velioğlu 4 , Thein An Trinh 1, 2 , Rong Wang 2, 5 , Jia Wei Chew 1, 2
Affiliation
|
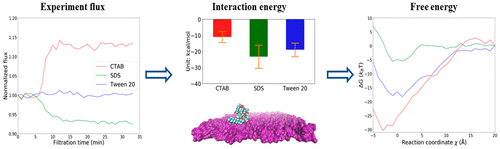
Membranes are effective for removing oil emulsions in oily wastewater treatments, which is important for environmental remediation as well as recovery of oil for economic benefits. Surfactants play a critical role in stabilizing the oil emulsions, but their effects on the inevitable membrane fouling phenomena remain poorly understood. The focus here is the interesting flux enhancement relative to water conferred by the cationic cetyltrimethylammonium bromide (CTAB) surfactant. Molecular dynamics simulations were performed to understand the interactions between three different surfactants and a hydrophilic polyvinylidene fluoride (PVDF) surface. Unbiased MD simulations quantify the surfactant–water, surfactant–membrane, and water–membrane interactions, but none appears well-correlated to the relative flux trends due to the interplay of all three interactions. To account for all interactions concurrently, umbrella sampling was performed to obtain the potential of mean force (PMF) curves. The adsorption of all three surfactants is driven by enthalpy (rather than entropy), and CTAB was found to have the most attractive binding free energy, smallest equilibrium distance, and looser water network near the PVDF surface, which are tied to the experimental observation of flux enhancement and highest retention. The Angstrom-scale results here reveal the need to consider all the interactions simultaneously rather than separately to fully account for mechanisms underlying membrane fouling by surfactants.
中文翻译:

使用分子动力学模拟和伞采样研究表面活性剂 - 膜相互作用
膜可有效去除含油废水处理中的油乳液,这对于环境修复和油回收以获得经济效益非常重要。表面活性剂在稳定油乳液方面起着关键作用,但它们对不可避免的膜污染现象的影响仍然知之甚少。这里的重点是由阳离子十六烷基三甲基溴化铵 (CTAB) 表面活性剂赋予的相对于水的有趣通量增强。进行分子动力学模拟以了解三种不同表面活性剂与亲水性聚偏二氟乙烯 (PVDF) 表面之间的相互作用。无偏 MD 模拟量化了表面活性剂 - 水、表面活性剂 - 膜和水 - 膜相互作用,但由于所有三种相互作用的相互作用,似乎没有一个与相对通量趋势密切相关。为了同时考虑所有相互作用,进行伞状采样以获得平均力 (PMF) 曲线的潜力。所有三种表面活性剂的吸附都是由焓(而不是熵)驱动的,并且发现 CTAB 具有最具吸引力的结合自由能、最小的平衡距离和靠近 PVDF 表面的较松散的水网络,这与实验观察有关通量增强和最高保留。这里的埃尺度结果表明需要同时考虑所有相互作用而不是单独考虑表面活性剂污染膜的潜在机制。进行伞状采样以获得平均力 (PMF) 曲线的潜力。所有三种表面活性剂的吸附都是由焓(而不是熵)驱动的,并且发现 CTAB 具有最具吸引力的结合自由能、最小的平衡距离和靠近 PVDF 表面的较松散的水网络,这与实验观察有关通量增强和最高保留。这里的埃尺度结果表明需要同时考虑所有相互作用而不是单独考虑表面活性剂污染膜的潜在机制。进行伞状采样以获得平均力 (PMF) 曲线的潜力。所有三种表面活性剂的吸附都是由焓(而不是熵)驱动的,并且发现 CTAB 具有最具吸引力的结合自由能、最小的平衡距离和靠近 PVDF 表面的较松散的水网络,这与实验观察有关通量增强和最高保留。这里的埃尺度结果表明需要同时考虑所有相互作用而不是单独考虑表面活性剂污染膜的潜在机制。PVDF 表面附近较松散的水网络,这与通量增强和最高保留率的实验观察有关。这里的埃尺度结果表明需要同时考虑所有相互作用而不是单独考虑表面活性剂污染膜的潜在机制。PVDF 表面附近较松散的水网络,这与通量增强和最高保留率的实验观察有关。这里的埃尺度结果表明需要同时考虑所有相互作用而不是单独考虑表面活性剂污染膜的潜在机制。
更新日期:2021-10-08
中文翻译:

使用分子动力学模拟和伞采样研究表面活性剂 - 膜相互作用
膜可有效去除含油废水处理中的油乳液,这对于环境修复和油回收以获得经济效益非常重要。表面活性剂在稳定油乳液方面起着关键作用,但它们对不可避免的膜污染现象的影响仍然知之甚少。这里的重点是由阳离子十六烷基三甲基溴化铵 (CTAB) 表面活性剂赋予的相对于水的有趣通量增强。进行分子动力学模拟以了解三种不同表面活性剂与亲水性聚偏二氟乙烯 (PVDF) 表面之间的相互作用。无偏 MD 模拟量化了表面活性剂 - 水、表面活性剂 - 膜和水 - 膜相互作用,但由于所有三种相互作用的相互作用,似乎没有一个与相对通量趋势密切相关。为了同时考虑所有相互作用,进行伞状采样以获得平均力 (PMF) 曲线的潜力。所有三种表面活性剂的吸附都是由焓(而不是熵)驱动的,并且发现 CTAB 具有最具吸引力的结合自由能、最小的平衡距离和靠近 PVDF 表面的较松散的水网络,这与实验观察有关通量增强和最高保留。这里的埃尺度结果表明需要同时考虑所有相互作用而不是单独考虑表面活性剂污染膜的潜在机制。进行伞状采样以获得平均力 (PMF) 曲线的潜力。所有三种表面活性剂的吸附都是由焓(而不是熵)驱动的,并且发现 CTAB 具有最具吸引力的结合自由能、最小的平衡距离和靠近 PVDF 表面的较松散的水网络,这与实验观察有关通量增强和最高保留。这里的埃尺度结果表明需要同时考虑所有相互作用而不是单独考虑表面活性剂污染膜的潜在机制。进行伞状采样以获得平均力 (PMF) 曲线的潜力。所有三种表面活性剂的吸附都是由焓(而不是熵)驱动的,并且发现 CTAB 具有最具吸引力的结合自由能、最小的平衡距离和靠近 PVDF 表面的较松散的水网络,这与实验观察有关通量增强和最高保留。这里的埃尺度结果表明需要同时考虑所有相互作用而不是单独考虑表面活性剂污染膜的潜在机制。PVDF 表面附近较松散的水网络,这与通量增强和最高保留率的实验观察有关。这里的埃尺度结果表明需要同时考虑所有相互作用而不是单独考虑表面活性剂污染膜的潜在机制。PVDF 表面附近较松散的水网络,这与通量增强和最高保留率的实验观察有关。这里的埃尺度结果表明需要同时考虑所有相互作用而不是单独考虑表面活性剂污染膜的潜在机制。


























 京公网安备 11010802027423号
京公网安备 11010802027423号