Progress in Neurobiology ( IF 6.7 ) Pub Date : 2021-08-26 , DOI: 10.1016/j.pneurobio.2021.102157 Shaily Malik 1 , Silvana Valdebenito 2 , Daniela D'Amico 2 , Brendan Prideaux 2 , Eliseo A Eugenin 2
|
HIV-associated neurological dysfunction is observed in more than half of the HIV-infected population, even in the current antiretroviral era. The mechanisms by which HIV mediates CNS dysfunction are not well understood but have been associated with the presence of long-lasting HIV reservoirs. In the CNS, macrophage/microglia and a small population of astrocytes harbor the virus. However, the low number of HIV-infected cells does not correlate with the high degree of damage, suggesting that mechanisms of damage amplification may be involved.
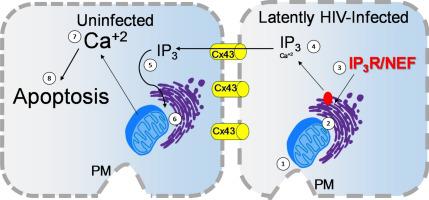
Here, we demonstrate that the survival mechanism of HIV-infected cells and the apoptosis of surrounding uninfected cells is regulated by inter-organelle interactions among the mitochondria/Golgi/endoplasmic reticulum system and the associated signaling mediated by IP3 and calcium. We identified that latently HIV-infected astrocytes had elevated intracellular levels of IP3, a master regulator second messenger, which diffuses via gap junctions into neighboring uninfected astrocytes resulting in their apoptosis. In addition, using laser capture microdissection, we confirmed that bystander apoptosis of uninfected astrocytes and the survival of HIV-infected astrocytes were dependent on mitochondrial function, intracellular calcium, and IP3 signaling. Blocking gap junction channels did not prevent an increase in IP3 or inter-organelle dysfunction in HIV-infected cells but reduced the amplification of apoptosis into uninfected neighboring cells. Our data provide a mechanistic explanation for bystander damage induced by surviving infected cells that serve as viral reservoirs and provide potential targets for interventions to reduce the devastating consequences of HIV within the brain.
中文翻译:

星形胶质细胞的 HIV 感染危及细胞器间相互作用和肌醇磷酸代谢:旁观者损伤和病毒库存活的潜在机制
在超过一半的 HIV 感染人群中观察到 HIV 相关的神经功能障碍,即使在当前的抗逆转录病毒时代也是如此。HIV 介导 CNS 功能障碍的机制尚不清楚,但与长期 HIV 宿主的存在有关。在中枢神经系统中,巨噬细胞/小胶质细胞和一小部分星形胶质细胞携带病毒。然而,感染 HIV 的细胞数量少与损伤程度高无关,这表明可能涉及损伤放大机制。
在这里,我们证明了 HIV 感染细胞的存活机制和周围未感染细胞的凋亡受线粒体/高尔基体/内质网系统之间的细胞器间相互作用以及 IP 3和钙介导的相关信号传导的调节。我们发现,潜伏性 HIV 感染的星形胶质细胞细胞内 IP 3 水平升高,IP 3是一种主要的调节第二信使,它通过间隙连接扩散到相邻的未感染星形胶质细胞中,导致它们的凋亡。此外,使用激光捕获显微切割,我们证实未感染星形胶质细胞的旁观者凋亡和 HIV 感染星形胶质细胞的存活取决于线粒体功能、细胞内钙和 IP 3发信号。阻断间隙连接通道并不能阻止HIV 感染细胞中 IP 3或细胞器间功能障碍的增加,但会减少细胞凋亡向未感染邻近细胞的放大。我们的数据为幸存的受感染细胞引起的旁观者损害提供了机械解释,这些受感染细胞充当病毒库,并为干预措施提供了潜在目标,以减少大脑内 HIV 的破坏性后果。


























 京公网安备 11010802027423号
京公网安备 11010802027423号