Journal of Molecular Biology ( IF 5.6 ) Pub Date : 2021-08-26 , DOI: 10.1016/j.jmb.2021.167217 Ashish Sethi 1 , Shoni Bruell 2 , Tim Ryan 3 , Fei Yan 1 , Mohammad Hossein Tanipour 1 , Yee-Foong Mok 4 , Chris Draper-Joyce 5 , Yogesh Khandokar 1 , Riley D Metcalfe 1 , Michael D W Griffin 1 , Daniel J Scott 2 , Mohammad Akhter Hossain 6 , Emma J Petrie 7 , Ross A D Bathgate 2 , Paul R Gooley 1
|
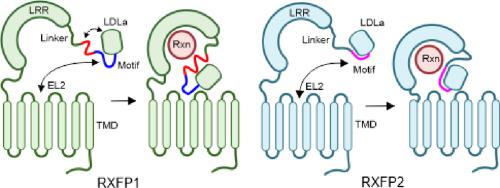
Our poor understanding of the mechanism by which the peptide-hormone H2 relaxin activates its G protein coupled receptor, RXFP1 and the related receptor RXFP2, has hindered progress in its therapeutic development. Both receptors possess large ectodomains, which bind H2 relaxin, and contain an N-terminal LDLa module that is essential for receptor signaling and postulated to be a tethered agonist. Here, we show that a conserved motif (GDxxGWxxxF), C-terminal to the LDLa module, is critical for receptor activity. Importantly, this motif adopts different structures in RXFP1 and RXFP2, suggesting distinct activation mechanisms. For RXFP1, the motif is flexible, weakly associates with the LDLa module, and requires H2 relaxin binding to stabilize an active conformation. Conversely, the GDxxGWxxxF motif in RXFP2 is more closely associated with the LDLa module, forming an essential binding interface for H2 relaxin. These differences in the activation mechanism will aid drug development targeting these receptors.
中文翻译:

对松弛素结合和栓系激动剂介导的 RXFP1 和 RXFP2 激活的独特模式的结构洞察
我们对肽激素 H2 松弛素激活其 G 蛋白偶联受体 RXFP1 和相关受体 RXFP2 的机制了解不足,阻碍了其治疗发展的进展。两种受体都具有大的胞外域,可结合 H2 松弛素,并包含 N 端 LDLa 模块,该模块对于受体信号传导至关重要,并被认为是一种束缚激动剂。在这里,我们展示了 LDLa 模块 C 端的保守基序 (GDxxGWxxxF) 对于受体活性至关重要。重要的是,这个基序在 RXFP1 和 RXFP2 中采用不同的结构,表明不同的激活机制。对于 RXFP1,其基序是灵活的,与 LDLa 模块弱关联,需要 H2 松弛素结合以稳定活性构象。反过来,RXFP2 中的 GDxxGWxxxF 基序与 LDLa 模块更密切相关,形成 H2 松弛素的必要结合界面。激活机制的这些差异将有助于针对这些受体的药物开发。


























 京公网安备 11010802027423号
京公网安备 11010802027423号