Journal of Energy Chemistry ( IF 13.1 ) Pub Date : 2021-08-26 , DOI: 10.1016/j.jechem.2021.08.045 Wei Zhang 1 , Fenfen Ma 2 , Sibei Guo 3 , Xin Chen 2 , Ziqi Zeng 1 , Qiang Wu 4 , Shuping Li 1 , Shijie Cheng 1 , Jia Xie 1
|
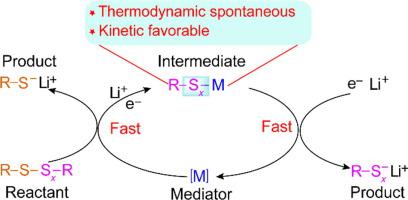
Organosulfides offer new opportunities for high performance lithium-sulfur (Li-S) batteries because of materials abundance, versatile structures and unique properties. Yet, their redox kinetics as well as cycling performance need to be further improved. Employing redox mediators is a highly effective strategy to address above challenges. However, the underlying mechanism in this chemistry is so far insufficiently explored. Here, phenyl disulfide (PhS–SPh) and phenyl diselenide (PhSe–SePh) are used as a model system for mechanistic understanding of organosulfide electrochemistry, particularly the rate acceleration. Profiling the reaction thermodynamics and charge-discharge process reveals redox of both S–S and C–S bonds, as well as that the coupling between radical exchange and electrochemical redox is the key to enhance the sulfur kinetics. This study not only establishes a basic understanding of orgaonsulfide electrochemistry in Li-S batteries, but also points out a general strategy for enhancing the kinetics of sulfur electrodes in electrochemical devices.
中文翻译:

用于锂有机硫化物电池中有机硫化物电化学机理研究的模型阴极
由于材料丰富、结构多样和独特的特性,有机硫化物为高性能锂硫 (Li-S) 电池提供了新的机会。然而,它们的氧化还原动力学以及循环性能需要进一步提高。使用氧化还原介质是解决上述挑战的高效策略。然而,到目前为止,这种化学反应的潜在机制还没有得到充分的探索。在这里,苯基二硫化物(PhS-SPh)和苯基二硒化物(PhSe-SePh)被用作模型系统,用于有机硫化物电化学的机理理解,特别是速率加速。分析反应热力学和充放电过程揭示了 S-S 和 C-S 键的氧化还原,以及自由基交换和电化学氧化还原之间的耦合是增强硫动力学的关键。



























 京公网安备 11010802027423号
京公网安备 11010802027423号