当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Adenylate Kinase-Catalyzed Reaction of AMP in Pieces: Enzyme Activation for Phosphoryl Transfer to Phosphite Dianion
Biochemistry ( IF 2.9 ) Pub Date : 2021-08-26 , DOI: 10.1021/acs.biochem.1c00535 Patrick L Fernandez 1 , John P Richard 1
Biochemistry ( IF 2.9 ) Pub Date : 2021-08-26 , DOI: 10.1021/acs.biochem.1c00535 Patrick L Fernandez 1 , John P Richard 1
Affiliation
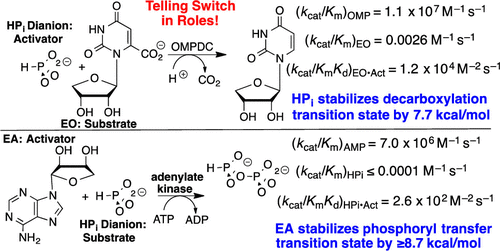
|
The binding of adenosine 5′-triphosphate (ATP) and adenosine 5′-monophosphate (AMP) to adenylate kinase (AdK) drives closure of lids over the substrate adenosyl groups. We test the hypothesis that this conformational change activates AdK for catalysis. The rate constants for Homo sapiens adenylate kinase 1 (HsAdK1)-catalyzed phosphoryl group transfer to AMP, kcat/Km = 7.0 × 106 M–1 s–1, and phosphite dianion, (kHPi)obs ≤1 × 10–4 M–1 s–1, show that the binding energy of the adenosyl group effects a ≥7.0 × 1010-fold rate acceleration of phosphoryl transfer from ATP. The third-order rate constant of kcat/KHPiKEA = 260 M–2 s–1 for 1-(β-d-erythrofuranosyl)adenine (EA)-activated phosphoryl transfer to phosphite dianion was determined, and the isohypophosphate reaction product characterized by 31P NMR. The results demonstrate the following: (i) a ≥14.7 kcal/mol stabilization of the transition state for phosphoryl transfer by the adenosyl group of AMP and a ≥2.6 × 106-fold rate acceleration from the EA-driven conformational change and (ii) the recovery of ≥8.7 kcal/mol of this transition state stabilization for EA-activated phosphoryl transfer from ATP to phosphite.
中文翻译:

腺苷酸激酶催化的 AMP 片段反应:磷酸转移至亚磷酸二价阴离子的酶活化
腺苷 5'-三磷酸 (ATP) 和腺苷 5'-单磷酸 (AMP) 与腺苷酸激酶 (AdK) 的结合驱动底物腺苷基上的盖子闭合。我们检验了这种构象变化激活 AdK 进行催化的假设。智人腺苷酸激酶 1 ( Hs AdK1) 催化的磷酰基转移到 AMP的速率常数k cat / K m = 7.0 × 10 6 M –1 s –1和亚磷酸二价阴离子 ( k HPi ) obs ≤1 × 10 –4 M –1秒–1,表明腺苷基团的结合能影响 ATP 磷酸转移的 ≥7.0 × 10 10倍速率加速。测定了 1-(β- d -赤呋喃糖基)腺嘌呤 (EA) 激活的磷酰基转移到亚磷酸二价阴离子的k cat / K HPi K EA = 260 M –2 s –1的三阶速率常数,并确定了异次磷酸盐反应产物经31 P NMR 表征。结果表明:(i) AMP 的腺苷基团转移磷酰基的过渡态稳定≥14.7 kcal/mol,≥2.6 × 10 6- EA驱动的构象变化的倍率加速和(ii)恢复≥8.7 kcal / mol的这种过渡态稳定化,用于EA激活的磷酸基从ATP到亚磷酸酯的转移。
更新日期:2021-09-07
中文翻译:

腺苷酸激酶催化的 AMP 片段反应:磷酸转移至亚磷酸二价阴离子的酶活化
腺苷 5'-三磷酸 (ATP) 和腺苷 5'-单磷酸 (AMP) 与腺苷酸激酶 (AdK) 的结合驱动底物腺苷基上的盖子闭合。我们检验了这种构象变化激活 AdK 进行催化的假设。智人腺苷酸激酶 1 ( Hs AdK1) 催化的磷酰基转移到 AMP的速率常数k cat / K m = 7.0 × 10 6 M –1 s –1和亚磷酸二价阴离子 ( k HPi ) obs ≤1 × 10 –4 M –1秒–1,表明腺苷基团的结合能影响 ATP 磷酸转移的 ≥7.0 × 10 10倍速率加速。测定了 1-(β- d -赤呋喃糖基)腺嘌呤 (EA) 激活的磷酰基转移到亚磷酸二价阴离子的k cat / K HPi K EA = 260 M –2 s –1的三阶速率常数,并确定了异次磷酸盐反应产物经31 P NMR 表征。结果表明:(i) AMP 的腺苷基团转移磷酰基的过渡态稳定≥14.7 kcal/mol,≥2.6 × 10 6- EA驱动的构象变化的倍率加速和(ii)恢复≥8.7 kcal / mol的这种过渡态稳定化,用于EA激活的磷酸基从ATP到亚磷酸酯的转移。


























 京公网安备 11010802027423号
京公网安备 11010802027423号