当前位置:
X-MOL 学术
›
ACS Earth Space Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Hydronium Ion Acidity Above and Below the Interface of Aqueous Microdroplets
ACS Earth and Space Chemistry ( IF 3.4 ) Pub Date : 2021-08-25 , DOI: 10.1021/acsearthspacechem.1c00067 Agustín J. Colussi 1 , Shinichi Enami 2 , Shinnosuke Ishizuka 2
ACS Earth and Space Chemistry ( IF 3.4 ) Pub Date : 2021-08-25 , DOI: 10.1021/acsearthspacechem.1c00067 Agustín J. Colussi 1 , Shinichi Enami 2 , Shinnosuke Ishizuka 2
Affiliation
|
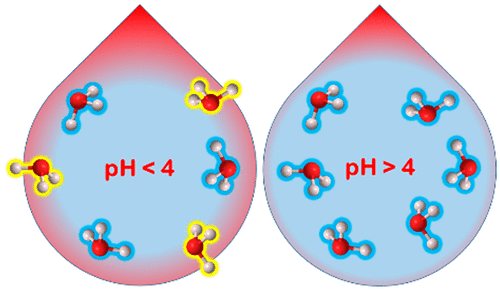
Atmospheric cloud, fog and aerosol microdroplets are more acidic than previously assumed. The fact that interfacial reactions on microdroplets are faster than anticipated has enhanced their role in atmospheric chemistry and raised the question of whether their interfaces are more or less acidic than the bulk phase. It turns out that acidity and its pH dependence sharply change across interfacial layers. Surface-specific experiments show that the protonations of gas-phase molecules at the outermost layer (OTL) of aqueous microdroplets are very different from those dissolved in deeper layers. Trimethylamine (TMA) is protonated, whereas the weak base isoprene (ISO) is not as expected, when dissolved in pH < pKa(TMA) = 9.8 microdroplets. In dramatic contrast, both gas-phase TMA and ISO are protonated at the OTL of pH < 4 microdroplets. Because ISO is only protonated in concentrated acids H3O+ ions at the OTL of pH < 4 microdroplets are superacidic. Conversely, the OTL of pH > 4 microdroplets lacks the H3O+ ions that protonate TMA in deeper layers. H3O+ ions become more acidic toward the surface (i.e., the free energies of proton transfer, H3O+ + B = H2O + BH+, become more negative) because hydration losses in lower density OTL water destabilize the small H3O+ ion relative to the larger protonated bases BH+. Because the OTL behaves as neutral at pH ∼ 4 (i.e., its pKw ∼ 8) interfacial water may be more dissociated than in the bulk. In short, the acidity of aqueous microdroplets probed by gas-phase molecules at the OTL is different from the acidity experienced by solutes in deeper layers and should not be confused with pH, which represents the uniform thermodynamic activity rather than the local acidities of H3O+ ions as a function of depth. These concepts should become standard in interfacial atmospheric chemistry.
中文翻译:

水微滴界面上方和下方的水合氢离子酸度
大气云、雾和气溶胶微滴的酸性比之前假设的要强。微滴上的界面反应比预期的要快这一事实增强了它们在大气化学中的作用,并提出了它们的界面是否比本体相酸性更多或更少的问题。事实证明,酸度及其 pH 依赖性在界面层之间急剧变化。表面特异性实验表明,水性微滴最外层 (OTL) 的气相分子的质子化与溶解在更深层的气相分子的质子化非常不同。当溶解在 pH < p K a 中时,三甲胺 (TMA) 被质子化,而弱碱异戊二烯 (ISO) 不像预期的那样(TMA) = 9.8 微滴。形成鲜明对比的是,气相 TMA 和 ISO 在 pH < 4 微滴的 OTL 下质子化。因为 ISO 仅在浓酸中质子化,所以 H 3 O +离子在 OTL 的 pH < 4 微滴是超酸性的。相反,pH > 4 微滴的 OTL 缺乏在更深层质子化 TMA的 H 3 O +离子。H 3 O +离子朝向表面变得更酸(即质子转移的自由能,H 3 O + + B = H 2 O + BH +,变得更负),因为低密度 OTL 水中的水合损失使小分子不稳定H 3 O+离子相对于较大的质子化碱基 BH +。因为 OTL 在 pH ~ 4(即它的 p K w~ 8)时表现为中性,界面水可能比本体更容易离解。简而言之,在 OTL 处由气相分子探测的水性微滴的酸度与更深层溶质所经历的酸度不同,不应与 pH 值混淆,后者代表了均匀的热力学活动,而不是 H 3的局部酸度O +离子作为深度的函数。这些概念应该成为界面大气化学的标准。
更新日期:2021-09-16
中文翻译:

水微滴界面上方和下方的水合氢离子酸度
大气云、雾和气溶胶微滴的酸性比之前假设的要强。微滴上的界面反应比预期的要快这一事实增强了它们在大气化学中的作用,并提出了它们的界面是否比本体相酸性更多或更少的问题。事实证明,酸度及其 pH 依赖性在界面层之间急剧变化。表面特异性实验表明,水性微滴最外层 (OTL) 的气相分子的质子化与溶解在更深层的气相分子的质子化非常不同。当溶解在 pH < p K a 中时,三甲胺 (TMA) 被质子化,而弱碱异戊二烯 (ISO) 不像预期的那样(TMA) = 9.8 微滴。形成鲜明对比的是,气相 TMA 和 ISO 在 pH < 4 微滴的 OTL 下质子化。因为 ISO 仅在浓酸中质子化,所以 H 3 O +离子在 OTL 的 pH < 4 微滴是超酸性的。相反,pH > 4 微滴的 OTL 缺乏在更深层质子化 TMA的 H 3 O +离子。H 3 O +离子朝向表面变得更酸(即质子转移的自由能,H 3 O + + B = H 2 O + BH +,变得更负),因为低密度 OTL 水中的水合损失使小分子不稳定H 3 O+离子相对于较大的质子化碱基 BH +。因为 OTL 在 pH ~ 4(即它的 p K w~ 8)时表现为中性,界面水可能比本体更容易离解。简而言之,在 OTL 处由气相分子探测的水性微滴的酸度与更深层溶质所经历的酸度不同,不应与 pH 值混淆,后者代表了均匀的热力学活动,而不是 H 3的局部酸度O +离子作为深度的函数。这些概念应该成为界面大气化学的标准。


























 京公网安备 11010802027423号
京公网安备 11010802027423号