当前位置:
X-MOL 学术
›
J. Cell. Physiol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Involvement of P2Y signaling in the restoration of glucose-induced insulin exocytosis in pancreatic β cells exposed to glucotoxicity
Journal of Cellular Physiology ( IF 5.6 ) Pub Date : 2021-08-25 , DOI: 10.1002/jcp.30564 Nour Mesto 1 , Danielle Bailbe 1 , Myriam Eskandar 1 , Gaëlle Pommier 1 , Stéphanie Gil 1, 2 , Stefania Tolu 1 , Jamileh Movassat 1 , Cécile Tourrel-Cuzin 1
Journal of Cellular Physiology ( IF 5.6 ) Pub Date : 2021-08-25 , DOI: 10.1002/jcp.30564 Nour Mesto 1 , Danielle Bailbe 1 , Myriam Eskandar 1 , Gaëlle Pommier 1 , Stéphanie Gil 1, 2 , Stefania Tolu 1 , Jamileh Movassat 1 , Cécile Tourrel-Cuzin 1
Affiliation
|
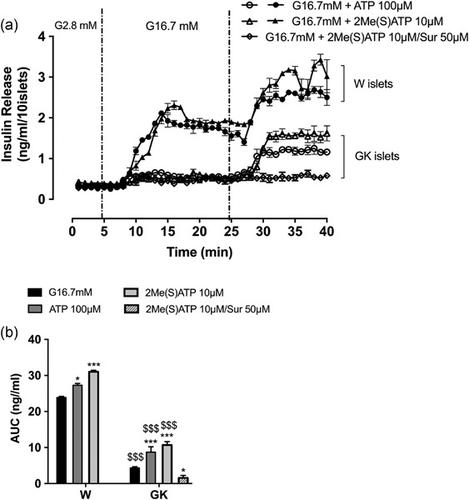
Purinergic P2Y receptors, by binding adenosine triphosphate (ATP), are known for enhancing glucose-stimulated insulin secretion (GSIS) in pancreatic β cells. However, the impact of these receptors in the actin dynamics and insulin granule exocytosis in these cells is not established, neither in normal nor in glucotoxic environment. In this study, we investigate the involvement of P2Y receptors on the behavior of insulin granules and the subcortical actin network dynamics in INS-1 832/13 β cells exposed to normal or glucotoxic environment and their role in GSIS. Our results show that the activation of P2Y purinergic receptors by ATP or its agonist increase the insulin granules exocytosis and the reorganization of the subcortical actin network and participate in the potentiation of GSIS. In addition, their activation in INS-1832/13 β-cells, with impaired insulin secretion following exposure to elevated glucose levels, restores GSIS competence through the distal steps of insulin exocytosis. These results are confirmed ex vivo by perifusion experiments on islets from type 2 diabetic (T2D) Goto-Kakizaki (GK) rats. Indeed, the P2Y receptor agonist restores the altered GSIS, which is normally lost in this T2D animal model. Moreover, we observed an improvement of the glucose tolerance, following the acute intraperitoneal injection of the P2Y agonist concomitantly with glucose, in diabetic GK rats. All these data provide new insights into the unprecedented therapeutic role of P2Y purinergic receptors in the pathophysiology of T2D.
中文翻译:

P2Y 信号通路参与恢复暴露于糖毒性的胰腺 β 细胞中葡萄糖诱导的胰岛素胞吐作用
嘌呤能 P2Y 受体通过结合三磷酸腺苷 (ATP) 来增强胰腺 β 细胞中葡萄糖刺激的胰岛素分泌 (GSIS)。然而,这些受体对这些细胞的肌动蛋白动力学和胰岛素颗粒胞吐作用的影响尚未确定,无论是在正常环境中还是在糖毒性环境中。在这项研究中,我们研究了 P2Y 受体对胰岛素颗粒行为和暴露于正常或糖毒性环境的 INS-1 832/13 β 细胞的皮层下肌动蛋白网络动力学的参与及其在 GSIS 中的作用。我们的研究结果表明,ATP 或其激动剂对 P2Y 嘌呤受体的激活增加了胰岛素颗粒的胞吐作用和皮层下肌动蛋白网络的重组,并参与了 GSIS 的增强。此外,它们在 INS-1832/13 β 细胞中的激活,在暴露于升高的葡萄糖水平后胰岛素分泌受损,通过胰岛素胞吐作用的远端步骤恢复 GSIS 能力。这些结果通过对来自 2 型糖尿病 (T2D) Goto-Kakizaki (GK) 大鼠的胰岛的灌注实验得到证实。事实上,P2Y 受体激动剂恢复了改变的 GSIS,这在 T2D 动物模型中通常会丢失。此外,我们观察到糖尿病 GK 大鼠在腹腔内急性注射 P2Y 激动剂和葡萄糖后,葡萄糖耐量有所改善。所有这些数据为 P2Y 嘌呤受体在 T2D 病理生理学中的前所未有的治疗作用提供了新的见解。这些结果通过对来自 2 型糖尿病 (T2D) Goto-Kakizaki (GK) 大鼠的胰岛的灌注实验得到证实。事实上,P2Y 受体激动剂恢复了改变的 GSIS,这在 T2D 动物模型中通常会丢失。此外,我们观察到糖尿病 GK 大鼠在腹腔内急性注射 P2Y 激动剂和葡萄糖后,葡萄糖耐量有所改善。所有这些数据为 P2Y 嘌呤受体在 T2D 病理生理学中的前所未有的治疗作用提供了新的见解。这些结果通过对来自 2 型糖尿病 (T2D) Goto-Kakizaki (GK) 大鼠的胰岛的灌注实验得到证实。事实上,P2Y 受体激动剂恢复了改变的 GSIS,这在 T2D 动物模型中通常会丢失。此外,我们观察到糖尿病 GK 大鼠在腹腔内急性注射 P2Y 激动剂和葡萄糖后,葡萄糖耐量有所改善。所有这些数据为 P2Y 嘌呤受体在 T2D 病理生理学中的前所未有的治疗作用提供了新的见解。在糖尿病 GK 大鼠中急性腹腔注射 P2Y 激动剂和葡萄糖后。所有这些数据为 P2Y 嘌呤受体在 T2D 病理生理学中的前所未有的治疗作用提供了新的见解。在糖尿病 GK 大鼠中急性腹腔注射 P2Y 激动剂和葡萄糖后。所有这些数据为 P2Y 嘌呤受体在 T2D 病理生理学中的前所未有的治疗作用提供了新的见解。
更新日期:2021-08-25
中文翻译:

P2Y 信号通路参与恢复暴露于糖毒性的胰腺 β 细胞中葡萄糖诱导的胰岛素胞吐作用
嘌呤能 P2Y 受体通过结合三磷酸腺苷 (ATP) 来增强胰腺 β 细胞中葡萄糖刺激的胰岛素分泌 (GSIS)。然而,这些受体对这些细胞的肌动蛋白动力学和胰岛素颗粒胞吐作用的影响尚未确定,无论是在正常环境中还是在糖毒性环境中。在这项研究中,我们研究了 P2Y 受体对胰岛素颗粒行为和暴露于正常或糖毒性环境的 INS-1 832/13 β 细胞的皮层下肌动蛋白网络动力学的参与及其在 GSIS 中的作用。我们的研究结果表明,ATP 或其激动剂对 P2Y 嘌呤受体的激活增加了胰岛素颗粒的胞吐作用和皮层下肌动蛋白网络的重组,并参与了 GSIS 的增强。此外,它们在 INS-1832/13 β 细胞中的激活,在暴露于升高的葡萄糖水平后胰岛素分泌受损,通过胰岛素胞吐作用的远端步骤恢复 GSIS 能力。这些结果通过对来自 2 型糖尿病 (T2D) Goto-Kakizaki (GK) 大鼠的胰岛的灌注实验得到证实。事实上,P2Y 受体激动剂恢复了改变的 GSIS,这在 T2D 动物模型中通常会丢失。此外,我们观察到糖尿病 GK 大鼠在腹腔内急性注射 P2Y 激动剂和葡萄糖后,葡萄糖耐量有所改善。所有这些数据为 P2Y 嘌呤受体在 T2D 病理生理学中的前所未有的治疗作用提供了新的见解。这些结果通过对来自 2 型糖尿病 (T2D) Goto-Kakizaki (GK) 大鼠的胰岛的灌注实验得到证实。事实上,P2Y 受体激动剂恢复了改变的 GSIS,这在 T2D 动物模型中通常会丢失。此外,我们观察到糖尿病 GK 大鼠在腹腔内急性注射 P2Y 激动剂和葡萄糖后,葡萄糖耐量有所改善。所有这些数据为 P2Y 嘌呤受体在 T2D 病理生理学中的前所未有的治疗作用提供了新的见解。这些结果通过对来自 2 型糖尿病 (T2D) Goto-Kakizaki (GK) 大鼠的胰岛的灌注实验得到证实。事实上,P2Y 受体激动剂恢复了改变的 GSIS,这在 T2D 动物模型中通常会丢失。此外,我们观察到糖尿病 GK 大鼠在腹腔内急性注射 P2Y 激动剂和葡萄糖后,葡萄糖耐量有所改善。所有这些数据为 P2Y 嘌呤受体在 T2D 病理生理学中的前所未有的治疗作用提供了新的见解。在糖尿病 GK 大鼠中急性腹腔注射 P2Y 激动剂和葡萄糖后。所有这些数据为 P2Y 嘌呤受体在 T2D 病理生理学中的前所未有的治疗作用提供了新的见解。在糖尿病 GK 大鼠中急性腹腔注射 P2Y 激动剂和葡萄糖后。所有这些数据为 P2Y 嘌呤受体在 T2D 病理生理学中的前所未有的治疗作用提供了新的见解。



























 京公网安备 11010802027423号
京公网安备 11010802027423号