当前位置:
X-MOL 学术
›
Biotechnol. Bioeng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Tuning enzymatic properties by protein engineering toward catalytic tetrad of carbonyl reductase
Biotechnology and Bioengineering ( IF 3.8 ) Pub Date : 2021-08-26 , DOI: 10.1002/bit.27925 Feng Cheng 1, 2, 3 , Qiu-Yao Zhai 1, 2, 3 , Xiao-Fan Gao 1, 2, 3 , Hua-Tao Liu 1, 2, 3 , Shuai Qiu 1, 2, 3 , Ya-Jun Wang 1, 2, 3 , Yu-Guo Zheng 1, 2, 3
Biotechnology and Bioengineering ( IF 3.8 ) Pub Date : 2021-08-26 , DOI: 10.1002/bit.27925 Feng Cheng 1, 2, 3 , Qiu-Yao Zhai 1, 2, 3 , Xiao-Fan Gao 1, 2, 3 , Hua-Tao Liu 1, 2, 3 , Shuai Qiu 1, 2, 3 , Ya-Jun Wang 1, 2, 3 , Yu-Guo Zheng 1, 2, 3
Affiliation
|
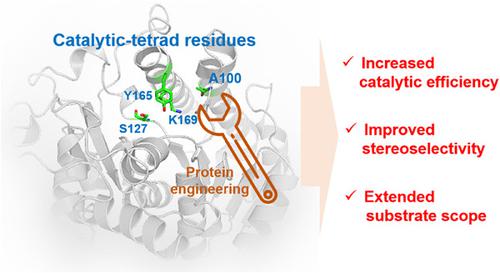
Enzyme engineering toward catalytic-tetrad residues usually results in activity loss. Unexpectedly, we found that a directed evolution campaign yielded a beneficial residue A100 in KmCR (a carbonyl reductase from Kluyveromyces marxianus ZJB14056), which is a residue of catalytic tetrad and conserved according to multiple sequence alignment. Inspired by this finding, we performed saturation mutagenesis on all the four residues of catalytic tetrad of KmCR. A number of variants with improved enzymatic activities were obtained. Among them, the variant KmCR_A100S exhibited increased catalytic efficiency (kcat/KM = 47.3 s−1·mM−1), improved stereoselectivity (from moderate selectivity (deP = 66.7%) to strict (S)-selectivity (deP > 99.5%)), and extended substrate scope, compared to those of KmCR_WT. In silico analysis showed that a relay system was rebuilt in KmCR via the beneficial residue S100. Furthermore, comparison of 11 protein engineering campaigns indicated that the beneficial position is easily overlooked due to the long distance (>10 Å) from ketone substrates. Since CRs share similar catalytic mechanism, the knowledge gained from this study has universal significance to CR engineering.
中文翻译:

通过蛋白质工程向羰基还原酶的催化四联体调整酶特性
针对催化四联体残基的酶工程通常会导致活性损失。出乎意料的是,我们发现定向进化运动在Km CR(来自马克斯克鲁维酵母 ZJB14056 的羰基还原酶)中产生了有益的残基 A100,它是催化四分体的残基,根据多序列比对是保守的。受这一发现的启发,我们对Km CR 的催化四分体的所有四个残基进行了饱和诱变。获得了许多具有改进的酶活性的变体。其中,变体Km CR_A100S表现出更高的催化效率(k cat /K M = 47.3 s -1 ·mM -1),与Km CR_WT相比,提高了立体选择性(从中等选择性 ( de P = 66.7%) 到严格 ( S ) -选择性 ( de P > 99.5%)),并扩大了底物范围。计算机分析表明,通过有益残基 S100在Km CR 中重建了一个中继系统。此外,对 11 种蛋白质工程活动的比较表明,由于与酮底物的距离很远(>10 Å),有益位置很容易被忽视。由于 CR 具有相似的催化机制,因此从这项研究中获得的知识对 CR 工程具有普遍意义。
更新日期:2021-08-26
中文翻译:

通过蛋白质工程向羰基还原酶的催化四联体调整酶特性
针对催化四联体残基的酶工程通常会导致活性损失。出乎意料的是,我们发现定向进化运动在Km CR(来自马克斯克鲁维酵母 ZJB14056 的羰基还原酶)中产生了有益的残基 A100,它是催化四分体的残基,根据多序列比对是保守的。受这一发现的启发,我们对Km CR 的催化四分体的所有四个残基进行了饱和诱变。获得了许多具有改进的酶活性的变体。其中,变体Km CR_A100S表现出更高的催化效率(k cat /K M = 47.3 s -1 ·mM -1),与Km CR_WT相比,提高了立体选择性(从中等选择性 ( de P = 66.7%) 到严格 ( S ) -选择性 ( de P > 99.5%)),并扩大了底物范围。计算机分析表明,通过有益残基 S100在Km CR 中重建了一个中继系统。此外,对 11 种蛋白质工程活动的比较表明,由于与酮底物的距离很远(>10 Å),有益位置很容易被忽视。由于 CR 具有相似的催化机制,因此从这项研究中获得的知识对 CR 工程具有普遍意义。


























 京公网安备 11010802027423号
京公网安备 11010802027423号