当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
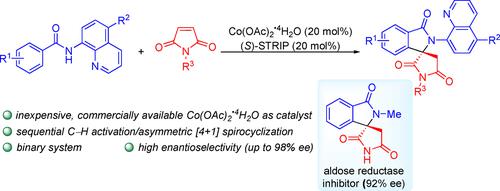
Synthesis of Chiral Spirolactams via Sequential C−H Olefination/Asymmetric [4+1] Spirocyclization under a Simple CoII/Chiral Spiro Phosphoric Acid Binary System
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2021-08-26 , DOI: 10.1002/anie.202108853 Wen-Kui Yuan 1 , Bing-Feng Shi 1, 2, 3
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2021-08-26 , DOI: 10.1002/anie.202108853 Wen-Kui Yuan 1 , Bing-Feng Shi 1, 2, 3
Affiliation
|
An unprecedented enantioselective synthesis of spiro-γ-lactams via a sequential C−H olefination/asymmetric [4+1] spirocyclization under a simple CoII/chiral spiro phosphoric acid (SPA) binary system is reported. A range of biologically important spiro-γ-lactams are obtained with high levels of enantioselectivity (up to 98 % ee). The concise, asymmetric synthesis of an aldose reductase inhibitor was successfully achieved. Notably, contrast to previous reports that relied on the use of cyclopentadienyl or its derivatives (achiral Cp*, CptBu, or chiral Cpx) ligated CoIII complexes requiring tedious steps to prepare, cheap and commercially available cobalt(II) acetate tetrahydrate was used as an efficient precatalyst.
中文翻译:

在简单的 CoII/手性螺磷酸二元体系下通过顺序 C-H 烯烃化/不对称 [4+1] 螺环化合成手性螺内酰胺
报道了在简单的 Co II /手性螺磷酸 (SPA) 二元体系下,通过连续的 C-H 烯化/不对称 [4+1] 螺环化,对螺-γ-内酰胺进行了前所未有的对映选择性合成。以高水平的对映选择性(高达 98% ee)获得了一系列生物学上重要的螺-γ-内酰胺。成功实现了醛糖还原酶抑制剂的简洁、不对称合成。值得注意的是,与之前依赖使用环戊二烯基或其衍生物(非手性 Cp*、Cp tBu或手性 Cp x)连接的 Co III 的报告相反 需要繁琐步骤来制备的复合物,廉价且可商购的四水合乙酸钴 (II) 被用作有效的预催化剂。
更新日期:2021-10-12
中文翻译:

在简单的 CoII/手性螺磷酸二元体系下通过顺序 C-H 烯烃化/不对称 [4+1] 螺环化合成手性螺内酰胺
报道了在简单的 Co II /手性螺磷酸 (SPA) 二元体系下,通过连续的 C-H 烯化/不对称 [4+1] 螺环化,对螺-γ-内酰胺进行了前所未有的对映选择性合成。以高水平的对映选择性(高达 98% ee)获得了一系列生物学上重要的螺-γ-内酰胺。成功实现了醛糖还原酶抑制剂的简洁、不对称合成。值得注意的是,与之前依赖使用环戊二烯基或其衍生物(非手性 Cp*、Cp tBu或手性 Cp x)连接的 Co III 的报告相反 需要繁琐步骤来制备的复合物,廉价且可商购的四水合乙酸钴 (II) 被用作有效的预催化剂。


























 京公网安备 11010802027423号
京公网安备 11010802027423号