Redox Biology ( IF 11.4 ) Pub Date : 2021-08-25 , DOI: 10.1016/j.redox.2021.102115 Shan Jiang 1 , Yongjie Shui 2 , Yu Cui 3 , Chun Tang 4 , Xiaohua Wang 4 , Xingyu Qiu 5 , Weipeng Hu 5 , Lingyan Fei 4 , Yun Li 6 , Suping Zhang 5 , Liang Zhao 5 , Nan Xu 5 , Fang Dong 5 , Xiaoqiu Ren 2 , Ruisheng Liu 7 , Pontus B Persson 8 , Andreas Patzak 8 , En Yin Lai 9 , Qichun Wei 2 , Zhihua Zheng 4
|
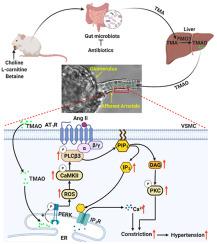
Gut microbiota produce Trimethylamine N-oxide (TMAO) by metabolizing dietary phosphatidylcholine, choline, l-carnitine and betaine. TMAO is implicated in the pathogenesis of chronic kidney disease (CKD), diabetes, obesity and atherosclerosis. We test, whether TMAO augments angiotensin II (Ang II)-induced vasoconstriction and hence promotes Ang II-induced hypertension. Plasma TMAO levels were indeed elevated in hypertensive patients, thus the potential pathways by which TMAO mediates these effects were explored. Ang II (400 ng/kg−1min−1) was chronically infused for 14 days via osmotic minipumps in C57Bl/6 mice. TMAO (1%) or antibiotics were given via drinking water. Vasoconstriction of renal afferent arterioles and mesenteric arteries were assessed by microperfusion and wire myograph, respectively. In Ang II-induced hypertensive mice, TMAO elevated systolic blood pressure and caused vasoconstriction, which was alleviated by antibiotics. TMAO enhanced the Ang II-induced acute pressor responses (12.2 ± 1.9 versus 20.6 ± 1.4 mmHg; P < 0.05) and vasoconstriction (32.3 ± 2.6 versus 55.9 ± 7.0%, P < 0.001). Ang II-induced intracellular Ca2+ release in afferent arterioles (147 ± 7 versus 234 ± 26%; P < 0.001) and mouse vascular smooth muscle cells (VSMC, 123 ± 3 versus 157 ± 9%; P < 0.001) increased by TMAO treatment. Preincubation of VSMC with TMAO activated the PERK/ROS/CaMKII/PLCβ3 pathway. Pharmacological inhibition of PERK, ROS, CaMKII and PLCβ3 impaired the effect of TMAO on Ca2+ release. Thus, TMAO facilitates Ang II-induced vasoconstriction, thereby promoting Ang II-induced hypertension, which involves the PERK/ROS/CaMKII/PLCβ3 axis.
中文翻译:

肠道微生物群依赖的三甲胺 N-氧化物加重血管紧张素 II 诱导的高血压
肠道微生物通过代谢膳食磷脂酰胆碱,胆碱,产生三甲胺N-氧化物(TMAO)升肉碱和甜菜碱。TMAO 与慢性肾病 (CKD)、糖尿病、肥胖症和动脉粥样硬化的发病机制有关。我们测试了 TMAO 是否会增强血管紧张素 II (Ang II) 诱导的血管收缩,从而促进 Ang II 诱导的高血压。高血压患者的血浆 TMAO 水平确实升高,因此探索了 TMAO 介导这些影响的潜在途径。Ang II (400 ng/kg -1 min -1) 通过 C57Bl/6 小鼠的渗透微型泵长期输注 14 天。通过饮用水给予 TMAO (1%) 或抗生素。分别通过微灌注和线肌描记器评估肾传入小动脉和肠系膜动脉的血管收缩。在 Ang II 诱导的高血压小鼠中,TMAO 会升高收缩压并引起血管收缩,而抗生素可缓解这种情况。TMAO 增强了血管紧张素 II 诱导的急性升压反应(12.2 ± 1.9 对 20.6 ± 1.4 mmHg;P < 0.05)和血管收缩(32.3 ± 2.6 对 55.9 ± 7.0%,P < 0.001)。Ang II 诱导的传入小动脉细胞内 Ca 2+释放(147 ± 7 与 234 ± 26%;P < 0.001)和小鼠血管平滑肌细胞(VSMC,123 ± 3 与 157 ± 9%;P < 0.001)) 通过 TMAO 处理增加。VSMC 与 TMAO 的预孵育激活了 PERK/ROS/CaMKII/PLCβ3 通路。PERK、ROS、CaMKII 和 PLCβ3 的药理学抑制削弱了 TMAO 对 Ca 2+释放的影响。因此,TMAO促进Ang II诱导的血管收缩,从而促进Ang II诱导的高血压,这涉及PERK/ROS/CaMKII/PLCβ3轴。


























 京公网安备 11010802027423号
京公网安备 11010802027423号