Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Inherently chiral dialkyloxy-calix[4]arene acetic acids as enantiodiscriminating additives for high-performance liquid chromatography separation of d,l-amino acids
Chirality ( IF 2 ) Pub Date : 2021-08-25 , DOI: 10.1002/chir.23355 Olga I Kalchenko 1 , Oleksandr O Trybrat 1 , Oleksandr A Yesypenko 1 , Viktoriya V Dyakonenko 2 , Svitlana V Shishkina 2 , Vitali I Kalchenko 1
Chirality ( IF 2 ) Pub Date : 2021-08-25 , DOI: 10.1002/chir.23355 Olga I Kalchenko 1 , Oleksandr O Trybrat 1 , Oleksandr A Yesypenko 1 , Viktoriya V Dyakonenko 2 , Svitlana V Shishkina 2 , Vitali I Kalchenko 1
Affiliation
|
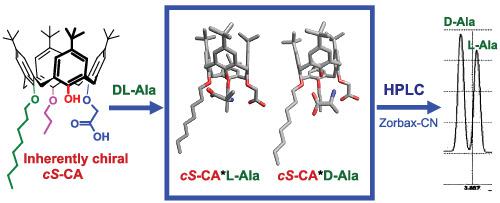
Inherently chiral dialkyloxy-calix[4]arene acetic acids with asymmetric placement of substituents on the lower rim of the macrocycle were first studied as enantiodiscriminating additives to the mobile phase MeCN/H2O/HCOOH (75/25/0.02 by volume) in the high-performance liquid chromatography (HPLC) separation of d,l-alanine and d,l-valine on the achiral stationary phase ZORBAX Original CN. The dependence of enantio-binding properties on the position of alkyl groups is demonstrated. The highest resolution (1.65) and enantioselectivity (1.80) were obtained for the 1,2-dipropyloxy-calix[4]arene acetic acid.
中文翻译:

固有手性二烷氧基杯[4]芳烃乙酸作为对映体鉴别添加剂用于 d,l-氨基酸的高效液相色谱分离
作为流动相 MeCN/H 2 O/HCOOH(体积比为 75/25/0.02)的对映体鉴别添加剂,首先研究了在大环下缘不对称放置取代基的固有手性二烷氧基杯[4]芳烃乙酸。 d , l-丙氨酸和d , l-缬氨酸在非手性固定相ZORBAX Original CN上的高效液相色谱(HPLC)分离。证明了对映体结合性质对烷基位置的依赖性。对于 1,2-二丙氧基-杯[4]芳烃乙酸,获得了最高分辨率 (1.65) 和对映选择性 (1.80)。
更新日期:2021-09-17
中文翻译:

固有手性二烷氧基杯[4]芳烃乙酸作为对映体鉴别添加剂用于 d,l-氨基酸的高效液相色谱分离
作为流动相 MeCN/H 2 O/HCOOH(体积比为 75/25/0.02)的对映体鉴别添加剂,首先研究了在大环下缘不对称放置取代基的固有手性二烷氧基杯[4]芳烃乙酸。 d , l-丙氨酸和d , l-缬氨酸在非手性固定相ZORBAX Original CN上的高效液相色谱(HPLC)分离。证明了对映体结合性质对烷基位置的依赖性。对于 1,2-二丙氧基-杯[4]芳烃乙酸,获得了最高分辨率 (1.65) 和对映选择性 (1.80)。


























 京公网安备 11010802027423号
京公网安备 11010802027423号