当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Time-Dependent Diastereodivergent Michael Addition Enabled by Phosphazenes Acting as Catalysts and Reactants
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2021-08-24 , DOI: 10.1002/adsc.202100570 Hamidulla Tukhtaev 1 , Stanislav Bezzubov 1 , Elena Tarasenko 1 , Mikhail Melnikov 1 , Konstantin Ivanov 2 , Ekaterina Budynina 3
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2021-08-24 , DOI: 10.1002/adsc.202100570 Hamidulla Tukhtaev 1 , Stanislav Bezzubov 1 , Elena Tarasenko 1 , Mikhail Melnikov 1 , Konstantin Ivanov 2 , Ekaterina Budynina 3
Affiliation
|
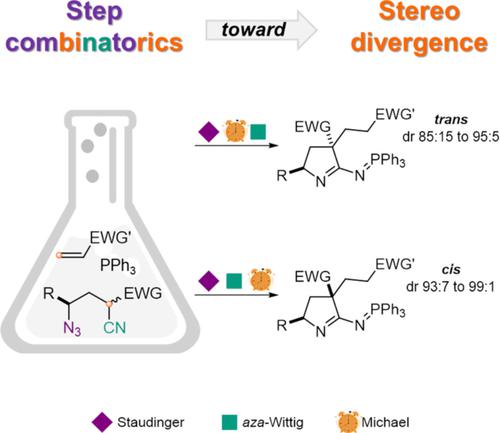
A switching of the step order within cascade process enabled design of a diastereodivergent approach to phosphazenopyrrolines with a 1,3-relationship of stereocenters. The approach is based on a cascade transformation of readily accessible γ-azidobutyronitriles that includes Staudinger, aza-Wittig and Michael steps. Stereodivergence is achieved at the Michael step that is self-catalyzed by phosphazene intermediates and provides construction of quaternary stereocenter. Depending on the type of phosphazene intermediates, generating either via Staudinger reaction or via subsequent intramolecular aza-Wittig reaction with nitrile and then reacting with a Michael acceptor, either trans- or cis-isomers of the target product can be produced. Thus, diastereoselectivity can be achieved by varying the time of addition for the Michael acceptor. Therefore, three distinct roles were revealed for phosphazenes in this process: catalysts, reactants and stereoselectivity controllers. This stereodivergent strategy was applied for the synthesis of all stereoisomers of the biorelevant tetrahydro-7-aza-indoles.
中文翻译:

由磷腈作为催化剂和反应物实现的时间依赖性非对映发散迈克尔加成
级联过程中步骤顺序的切换使得能够设计非对映发散方法来处理具有 1,3-立体中心关系的磷腈基吡咯啉。该方法基于易于获得的γ-叠氮丁腈的级联转化,包括 Staudinger、aza- Wittig和 Michael 步骤。立体发散是在迈克尔步骤实现的,由磷腈中间体自催化并提供四元立体中心的构建。根据磷腈中间体的类型,通过施陶丁格反应或通过随后的分子内氮杂-维蒂希与腈反应生成,然后与迈克尔受体反应,或者反式可以产生目标产物的- 或顺式异构体。因此,可以通过改变迈克尔受体的添加时间来实现非对映选择性。因此,磷腈在该过程中具有三个不同的作用:催化剂、反应物和立体选择性控制剂。这种立体发散策略被应用于生物相关四氢-7-氮杂-吲哚的所有立体异构体的合成。
更新日期:2021-08-24
中文翻译:

由磷腈作为催化剂和反应物实现的时间依赖性非对映发散迈克尔加成
级联过程中步骤顺序的切换使得能够设计非对映发散方法来处理具有 1,3-立体中心关系的磷腈基吡咯啉。该方法基于易于获得的γ-叠氮丁腈的级联转化,包括 Staudinger、aza- Wittig和 Michael 步骤。立体发散是在迈克尔步骤实现的,由磷腈中间体自催化并提供四元立体中心的构建。根据磷腈中间体的类型,通过施陶丁格反应或通过随后的分子内氮杂-维蒂希与腈反应生成,然后与迈克尔受体反应,或者反式可以产生目标产物的- 或顺式异构体。因此,可以通过改变迈克尔受体的添加时间来实现非对映选择性。因此,磷腈在该过程中具有三个不同的作用:催化剂、反应物和立体选择性控制剂。这种立体发散策略被应用于生物相关四氢-7-氮杂-吲哚的所有立体异构体的合成。



























 京公网安备 11010802027423号
京公网安备 11010802027423号