当前位置:
X-MOL 学术
›
ACS Earth Space Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Implementing Heterogeneous Crystal Surface Reactivity in Reactive Transport Simulations: The Example of Calcite Dissolution
ACS Earth and Space Chemistry ( IF 3.4 ) Pub Date : 2021-08-25 , DOI: 10.1021/acsearthspacechem.1c00099 Lotfollah Karimzadeh 1 , Cornelius Fischer 1
ACS Earth and Space Chemistry ( IF 3.4 ) Pub Date : 2021-08-25 , DOI: 10.1021/acsearthspacechem.1c00099 Lotfollah Karimzadeh 1 , Cornelius Fischer 1
Affiliation
|
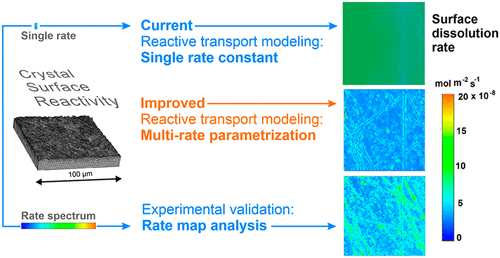
Both surface reactivity and fluid dynamics control the dissolution kinetics of crystalline material. In this study, we performed a 3D reactive transport simulation to investigate the impact of surface topography heterogeneity superimposed to fluid transport heterogeneity on the dissolution rate of calcite. The model simulates the chemical reaction of calcite dissolution, solute transport, and crystal surface geometry evolution. Importantly, we introduce heterogeneous surface reactivity into the reactive transport simulation. We test the surface slope factor as a proxy value for the intrinsic surface reactivity of dissolving crystal surface nanotopographies. Experimental data sets collected using vertical scanning interferometry validate this approach. The novel parametrization allows for the simulation of surface-controlled heterogeneous reactivity in reactive transport simulations of mineral surface dissolution.
中文翻译:

在反应输运模拟中实现异质晶体表面反应性:方解石溶解的例子
表面反应性和流体动力学都控制着结晶材料的溶解动力学。在这项研究中,我们进行了 3D 反应输运模拟,以研究叠加在流体输运异质性上的表面形貌异质性对方解石溶解速率的影响。该模型模拟方解石溶解、溶质迁移和晶体表面几何演化的化学反应。重要的是,我们在反应输运模拟中引入了异质表面反应性。我们测试表面斜率因子作为溶解晶体表面纳米形貌的内在表面反应性的代理值。使用垂直扫描干涉测量法收集的实验数据集验证了这种方法。
更新日期:2021-09-16
中文翻译:

在反应输运模拟中实现异质晶体表面反应性:方解石溶解的例子
表面反应性和流体动力学都控制着结晶材料的溶解动力学。在这项研究中,我们进行了 3D 反应输运模拟,以研究叠加在流体输运异质性上的表面形貌异质性对方解石溶解速率的影响。该模型模拟方解石溶解、溶质迁移和晶体表面几何演化的化学反应。重要的是,我们在反应输运模拟中引入了异质表面反应性。我们测试表面斜率因子作为溶解晶体表面纳米形貌的内在表面反应性的代理值。使用垂直扫描干涉测量法收集的实验数据集验证了这种方法。


























 京公网安备 11010802027423号
京公网安备 11010802027423号