当前位置:
X-MOL 学术
›
ACS Appl. Bio Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Efficient DNA Condensation Induced by Chiral β-Amino Acid-Based Cationic Surfactants
ACS Applied Bio Materials ( IF 4.7 ) Pub Date : 2021-08-25 , DOI: 10.1021/acsabm.1c00683 Bernat Pi-Boleda 1 , Sravani Ramisetty 2 , Ona Illa 1 , Vicenç Branchadell 1 , Rita S Dias 2 , Rosa M Ortuño 1
ACS Applied Bio Materials ( IF 4.7 ) Pub Date : 2021-08-25 , DOI: 10.1021/acsabm.1c00683 Bernat Pi-Boleda 1 , Sravani Ramisetty 2 , Ona Illa 1 , Vicenç Branchadell 1 , Rita S Dias 2 , Rosa M Ortuño 1
Affiliation
|
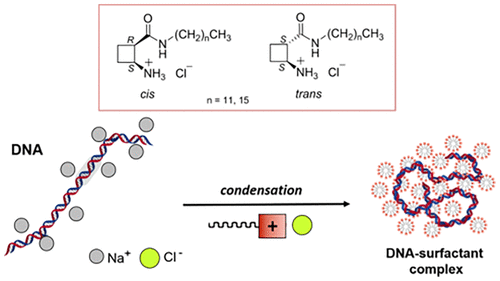
Four cationic chiral amino acid-based surfactants, cis- and trans-1 and cis- and trans-2, have been studied as DNA-condensing agents with enhanced properties and the absence of cell toxicity. The polar head of the surfactant is made of a cyclobutane β-amino acid in which the amino group is a hydrochloride salt and the carboxyl group is involved in an amide bond, allowing the link with hydrophobic C12 (surfactant 1) or C16 (surfactant 2) chains. The ability of these surfactants to condense DNA was investigated using a dye exclusion assay, gel electrophoresis, and circular dichroism and compared with the well-studied dodecyltrimethylammonium bromide (DTAB) and cetyltrimethylammonium bromide (CTAB). The surfactant with the longest chain length and the trans stereochemistry (trans-2) was found to be the most efficient in condensing the DNA, including CTAB. Surfactant cis-2 was found to be less efficient, probably due to its poorer solubility. The β-amino acid surfactants with the shorter chain length behaved similarly, such that the cis/trans stereochemistry does not seem to play a role in this case. Interestingly, these were also found to induce DNA condensation for the same concentration as trans-2 and CTAB but showed a lower binding cooperativity. Therefore, a longer alkyl chain only slightly improved the effectiveness of these surfactants. Further, atomic force microscopy revealed that they compact DNA into small complexes of about 55–110 nm in diameter.
中文翻译:

手性 β-氨基酸基阳离子表面活性剂诱导的高效 DNA 缩合
四种基于阳离子手性氨基酸的表面活性剂,顺式和反式- 1和顺式- 和反式- 2,已作为 DNA 缩合剂进行了研究,具有增强的特性和无细胞毒性。表面活性剂的极性头由环丁烷β-氨基酸组成,其中氨基为盐酸盐,羧基参与酰胺键,可与疏水性C 12(表面活性剂1)或C 16(表面活性剂2) 链。使用染料排除测定、凝胶电泳和圆二色性研究了这些表面活性剂凝聚 DNA 的能力,并与经过充分研究的十二烷基三甲基溴化铵 (DTAB) 和十六烷基三甲基溴化铵 (CTAB) 进行了比较。发现具有最长链长和反式立体化学 ( trans - 2 ) 的表面活性剂在缩合 DNA 方面最有效,包括 CTAB。发现表面活性剂cis - 2的效率较低,可能是由于其溶解度较差。具有较短链长的β-氨基酸表面活性剂表现相似,因此顺式/反式在这种情况下,立体化学似乎不起作用。有趣的是,还发现它们在与trans - 2和 CTAB相同浓度下诱导 DNA 凝聚,但显示出较低的结合协同性。因此,较长的烷基链仅略微提高了这些表面活性剂的有效性。此外,原子力显微镜显示它们将 DNA 压缩成直径约 55-110 nm 的小复合物。
更新日期:2021-09-20
中文翻译:

手性 β-氨基酸基阳离子表面活性剂诱导的高效 DNA 缩合
四种基于阳离子手性氨基酸的表面活性剂,顺式和反式- 1和顺式- 和反式- 2,已作为 DNA 缩合剂进行了研究,具有增强的特性和无细胞毒性。表面活性剂的极性头由环丁烷β-氨基酸组成,其中氨基为盐酸盐,羧基参与酰胺键,可与疏水性C 12(表面活性剂1)或C 16(表面活性剂2) 链。使用染料排除测定、凝胶电泳和圆二色性研究了这些表面活性剂凝聚 DNA 的能力,并与经过充分研究的十二烷基三甲基溴化铵 (DTAB) 和十六烷基三甲基溴化铵 (CTAB) 进行了比较。发现具有最长链长和反式立体化学 ( trans - 2 ) 的表面活性剂在缩合 DNA 方面最有效,包括 CTAB。发现表面活性剂cis - 2的效率较低,可能是由于其溶解度较差。具有较短链长的β-氨基酸表面活性剂表现相似,因此顺式/反式在这种情况下,立体化学似乎不起作用。有趣的是,还发现它们在与trans - 2和 CTAB相同浓度下诱导 DNA 凝聚,但显示出较低的结合协同性。因此,较长的烷基链仅略微提高了这些表面活性剂的有效性。此外,原子力显微镜显示它们将 DNA 压缩成直径约 55-110 nm 的小复合物。


























 京公网安备 11010802027423号
京公网安备 11010802027423号