Synthesis ( IF 2.6 ) Pub Date : 2021-08-23 , DOI: 10.1055/s-0037-1610781 Felipe C. Demidoff 1 , Leandro L. de Carvalho 2 , Eduardo José P. Rodrigues Filho 2 , Andréa Luzia F. de Souza 2 , Chaquip D. Netto 1
|
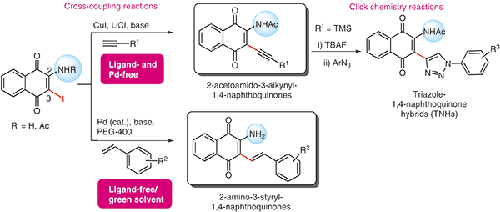
Functionalized 1,4-naphthoquinones have been employed as versatile synthons in organic synthesis, in addition to presenting a large array of biological activities. Herein, the applications of 2-amino-/ acetylamino-substituted 3-iodo-1,4-naphthoquinones in cross-coupling reactions are described to successfully afford sixteen novel 3-styryl-1,4-naphthoquinones (amino-stilbene-quinone hybrids) and four 3-alkynyl-1,4-naphthoquinone in overall good yields. Interestingly, the alkynylated derivatives could be obtained from ligand- and Pd-free CuI-mediated cross-coupling reactions, after extensive investigations to exclude Pd as a co-catalyst. Lastly, the desilanized terminal alkyne was subjected to click chemistry reactions to give two novel triazole-1,4-naphthoquinone hybrids.
中文翻译:

与 2-氨基-/乙酰氨基-取代的 3-Iodo-1,4-萘醌的交叉偶联反应:新型烯基和炔基萘醌及其衍生物的便捷合成
功能化的 1,4-萘醌除了具有大量的生物活性外,还被用作有机合成中的多功能合成子。在此,描述了 2-氨基-/乙酰氨基取代的 3-碘-1,4-萘醌在交叉偶联反应中的应用,成功提供了 16 种新型 3-苯乙烯基-1,4-萘醌(氨基-二苯乙烯-醌杂化物) ) 和四个 3-炔基-1,4-萘醌,总体收率良好。有趣的是,经过广泛研究以排除 Pd 作为助催化剂后,可以从无配体和无 Pd 的 Cu I介导的交叉偶联反应中获得炔基化衍生物。最后,对脱硅烷的末端炔进行点击化学反应,得到两种新型三唑-1,4-萘醌杂化物。



























 京公网安备 11010802027423号
京公网安备 11010802027423号