Journal of Electroanalytical Chemistry ( IF 4.5 ) Pub Date : 2021-08-24 , DOI: 10.1016/j.jelechem.2021.115630 Slađana Strmečki 1 , Lora Pereža 2
|
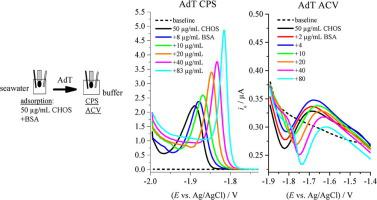
We investigated the electrochemical properties of the models chitosan oligosaccharide (CHOS), an amino-glycan, and BSA protein, as major components of N-bearing polymeric organic material (N-POM) in seawater. The chronopotentiometric stripping method (CPS) and a hanging mercury drop working electrode (HMDE) were used to detect catalytic peaks (Ep around –1.8 V/–1.9 V vs. Ag/AgCl (3M KCl)) generated by the catalytic hydrogen evolution reaction (CHER). “Peak HOS” of CHOS was not observed under seawater conditions (pH 8.2). Since both analytes measured separately produced catalytic peaks in CPS after their adsorptive transfer (AdT) from seawater into slightly acidic and neutral buffers (pH-s 5.3–7.1), we investigated measurement conditions that might allow selective AdT detection of CHOS in the CHOS + BSA seawater mixture. Only one catalytic peak was observed in the AdT CPS of mixture, which was attributed to the involvement of both compounds in adsorption on HMDE and consequently in CHER. The height and Ep of this peak tended to correspond to the analyte with the higher concentration in the mixture. Regardless of the choice of pH or buffer, the combination of CPS measurement conditions or the rinsing mode by relevant solvents and solutions during AdT, BSA was not efficiently removed from the HMDE and selective detection of CHOS in the mixture was not achieved. These results will be applicable in the electrochemical analysis of amino-glycans in natural seawater samples containing a mixture of N-POM, but also represent an investigation of the basic electrochemical properties of CHOS and BSA.
中文翻译:

海水条件下壳聚糖氨基-聚糖和BSA蛋白混合物的电化学
我们研究了模型壳寡糖的电化学性能(CHOS),氨基聚糖,和BSA蛋白,作为主要成分Ñ荷瘤在海水中的聚合物有机材料(N-POM)。计时电位汽提法 (CPS) 和悬汞滴工作电极 (HMDE) 用于检测催化析氢产生的催化峰(E p约 –1.8 V/–1.9 V vs. Ag/AgCl (3M KCl))反应 (CHER)。“峰值 H OS”的 CHOS 在海水条件下(pH 8.2)未观察到。由于两种分析物在从海水吸附转移 (AdT) 到微酸性和中性缓冲液 (pH-s 5.3–7.1) 后,分别测量的两种分析物在 CPS 中产生催化峰,因此我们研究了可能允许选择性 AdT 检测 CHOS + 中 CHOS 的测量条件BSA 海水混合物。在混合物的 AdT CPS 中仅观察到一个催化峰,这归因于两种化合物都参与了 HMDE 和 CHER 的吸附。高度和E p该峰的浓度往往对应于混合物中浓度较高的分析物。无论 pH 或缓冲液的选择,CPS 测量条件的组合或 AdT 期间相关溶剂和溶液的冲洗模式,BSA 都没有从 HMDE 中有效去除,并且没有实现混合物中 CHOS 的选择性检测。这些结果将适用于含有 N-POM 混合物的天然海水样品中氨基聚糖的电化学分析,也代表了对 CHOS 和 BSA 基本电化学性质的研究。


























 京公网安备 11010802027423号
京公网安备 11010802027423号