当前位置:
X-MOL 学术
›
J. Nat. Prod.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Indole Alkaloids from a Soil-Derived Clonostachys rosea
Journal of Natural Products ( IF 5.1 ) Pub Date : 2021-08-24 , DOI: 10.1021/acs.jnatprod.1c00457 Chun-Xiao Jiang 1, 2 , Bo Yu 1 , Ya-Mei Miao 1 , Hao Ren 1 , Qianhe Xu 1 , Chun Zhao 1, 3 , Li-Li Tian 1 , Zhen-Qing Yu 1 , Pan-Pan Zhou 1 , Xiaolei Wang 1 , Jianguo Fang 1 , Jiwen Zhang 3 , Jin Z Zhang 4 , Quan-Xiang Wu 1
Journal of Natural Products ( IF 5.1 ) Pub Date : 2021-08-24 , DOI: 10.1021/acs.jnatprod.1c00457 Chun-Xiao Jiang 1, 2 , Bo Yu 1 , Ya-Mei Miao 1 , Hao Ren 1 , Qianhe Xu 1 , Chun Zhao 1, 3 , Li-Li Tian 1 , Zhen-Qing Yu 1 , Pan-Pan Zhou 1 , Xiaolei Wang 1 , Jianguo Fang 1 , Jiwen Zhang 3 , Jin Z Zhang 4 , Quan-Xiang Wu 1
Affiliation
|
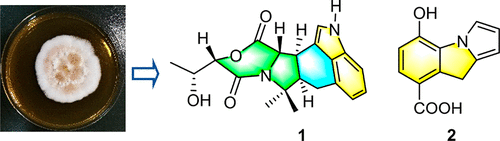
Clonorosins A (1) and B (2), two novel indole alkaloids featuring unprecedented 6/5/6/6/5 and 6/5/5 cores, together with seven known indole-linked 2,5-diketopiperazine alkaloids (3–9), were isolated from the soil-derived fungus Clonostachys rosea YRS-06. The new structures were proposed through HR-MS, NMR, and ECD spectroscopic data. They were established by comparing the calculated NMR, ECD, and specific rotation data with the experimental. To assist in determining the absolute configuration of the chiral carbon in the side chain of 2,5-diketopiperazine derivatives, flexible analogues 3i–3iv were synthesized and analyzed. 1 was active against Fusarium oxysporum with an MIC value of 50 μg/mL. 7 and 8 showed excellent activity against human HeLa and HepG2 cells with IC50 values of 0.12–0.60 μM.
中文翻译:

来自土壤的吲哚生物碱
Clonorosins A ( 1 ) 和 B ( 2 ),两种新型吲哚生物碱,具有前所未有的 6/5/6/6/5 和 6/5/5 核心,以及七种已知的吲哚连接的 2,5-二酮哌嗪生物碱 ( 3 – 9 ),从土壤衍生的真菌Clonostachys rosea YRS-06 中分离。新结构是通过 HR-MS、NMR 和 ECD 光谱数据提出的。它们是通过将计算的 NMR、ECD 和比旋转数据与实验数据进行比较来建立的。为了帮助确定 2,5-二酮哌嗪衍生物侧链中手性碳的绝对构型,合成并分析了柔性类似物3i – 3iv 。1对尖孢镰刀菌有活性,MIC 值为 50 μg/mL。图7和8显示了对人HeLa和HepG2细胞的优异活性,IC 50值为0.12-0.60 μM。
更新日期:2021-09-24
中文翻译:

来自土壤的吲哚生物碱
Clonorosins A ( 1 ) 和 B ( 2 ),两种新型吲哚生物碱,具有前所未有的 6/5/6/6/5 和 6/5/5 核心,以及七种已知的吲哚连接的 2,5-二酮哌嗪生物碱 ( 3 – 9 ),从土壤衍生的真菌Clonostachys rosea YRS-06 中分离。新结构是通过 HR-MS、NMR 和 ECD 光谱数据提出的。它们是通过将计算的 NMR、ECD 和比旋转数据与实验数据进行比较来建立的。为了帮助确定 2,5-二酮哌嗪衍生物侧链中手性碳的绝对构型,合成并分析了柔性类似物3i – 3iv 。1对尖孢镰刀菌有活性,MIC 值为 50 μg/mL。图7和8显示了对人HeLa和HepG2细胞的优异活性,IC 50值为0.12-0.60 μM。



























 京公网安备 11010802027423号
京公网安备 11010802027423号