当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Solubility Measurement, Preferential Solvation, and Solvent Effect of 3,5-Dinitrosalicylic Acid in Several Binary Aqueous Blends
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2021-08-23 , DOI: 10.1021/acs.jced.1c00396 Jiahong Chen 1 , Ali Farajtabar 2 , Abolghasem Jouyban 3 , William E. Acree 4 , Peizhi Zhu 1 , Hongkun Zhao 1
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2021-08-23 , DOI: 10.1021/acs.jced.1c00396 Jiahong Chen 1 , Ali Farajtabar 2 , Abolghasem Jouyban 3 , William E. Acree 4 , Peizhi Zhu 1 , Hongkun Zhao 1
Affiliation
|
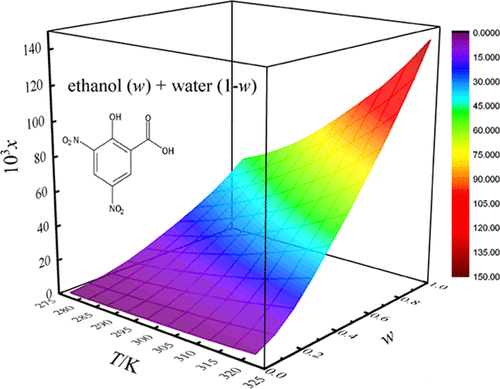
This research dealt with the equilibrium solubility, preferential solvation, and intra- and intermolecular interactions for 3,5-dinitrosalicylic acid dissolved in aqueous blends of N-methyl-2-pyrrolidinone (NMP)/ethanol/isopropanol/N,N-dimethylformamide (DMF) along with computational methodologies. All solubility experiments were performed using a shake-flask equilibration method under a pressure of 101.2 kPa from 278.15 to 323.15 K. For each aqueous blend, the minimum solubility value of 3,5-dinitrosalicylic acid was found in water at 278.15 K, and the maximum one was found in neat organic solvent at 323.15 K. The generated solubility was acceptably described by mixture response surface, van’t Hoff–Jouyban–Acree, modified Wilson and Jouyban–Acree model. The relative average deviations were all no larger than 5.57%. The analysis of linear solvation energy relationships demonstrated that the solvent descriptors of dipolarity-polarizability and solubility parameter were principally responsible for the 3,5-dinitrosalicylic acid solubility. Besides, the solvation of 3,5-dinitrosalicylic acid was studied quantitatively by inverse Kirkwood–Buff integrals. The positive values of preferential solvation parameter for 3,5-dinitrosalicylic acid in middle and cosolvent-rich mixtures strongly suggest that 3,5-dinitrosalicylic acid is preferentially solvated by NMP/ethanol/isopropanol/DMF. It could be conjectured that in these composition regions, 3,5-dinitrosalicylic acid served as Lewis acid in its interactions with surrounding NMP/ethanol/isopropanol/DMF molecules.
中文翻译:

3,5-二硝基水杨酸在几种二元水混合物中的溶解度测量、优先溶剂化和溶剂效应
本研究涉及溶解在N-甲基-2-吡咯烷酮 (NMP)/乙醇/异丙醇/ N , N 的水性混合物中的 3,5-二硝基水杨酸的平衡溶解度、优先溶剂化以及分子内和分子间相互作用-二甲基甲酰胺 (DMF) 以及计算方法。所有溶解度实验均使用摇瓶平衡法在 101.2 kPa 压力下从 278.15 到 323.15 K 进行。对于每种水性混合物,发现 3,5-二硝基水杨酸在水中的最小溶解度值为 278.15 K,并且在 323.15 K 的纯有机溶剂中发现了最大的一个。产生的溶解度可以通过混合物响应面、van't Hoff-Jouyban-Acree、修正的 Wilson 和 Jouyban-Acree 模型来描述。相对平均偏差均不大于5.57%。线性溶剂化能关系的分析表明,偶极-极化率和溶解度参数的溶剂描述符主要负责 3,5-二硝基水杨酸的溶解度。此外,3的溶解,5-二硝基水杨酸通过逆柯克伍德-布夫积分进行定量研究。中间和富含助溶剂的混合物中 3,5-二硝基水杨酸的优先溶剂化参数的正值强烈表明 3,5-二硝基水杨酸优先被 NMP/乙醇/异丙醇/DMF 溶剂化。可以推测,在这些组成区域中,3,5-二硝基水杨酸在与周围 NMP/乙醇/异丙醇/DMF 分子的相互作用中充当了路易斯酸。
更新日期:2021-09-09
中文翻译:

3,5-二硝基水杨酸在几种二元水混合物中的溶解度测量、优先溶剂化和溶剂效应
本研究涉及溶解在N-甲基-2-吡咯烷酮 (NMP)/乙醇/异丙醇/ N , N 的水性混合物中的 3,5-二硝基水杨酸的平衡溶解度、优先溶剂化以及分子内和分子间相互作用-二甲基甲酰胺 (DMF) 以及计算方法。所有溶解度实验均使用摇瓶平衡法在 101.2 kPa 压力下从 278.15 到 323.15 K 进行。对于每种水性混合物,发现 3,5-二硝基水杨酸在水中的最小溶解度值为 278.15 K,并且在 323.15 K 的纯有机溶剂中发现了最大的一个。产生的溶解度可以通过混合物响应面、van't Hoff-Jouyban-Acree、修正的 Wilson 和 Jouyban-Acree 模型来描述。相对平均偏差均不大于5.57%。线性溶剂化能关系的分析表明,偶极-极化率和溶解度参数的溶剂描述符主要负责 3,5-二硝基水杨酸的溶解度。此外,3的溶解,5-二硝基水杨酸通过逆柯克伍德-布夫积分进行定量研究。中间和富含助溶剂的混合物中 3,5-二硝基水杨酸的优先溶剂化参数的正值强烈表明 3,5-二硝基水杨酸优先被 NMP/乙醇/异丙醇/DMF 溶剂化。可以推测,在这些组成区域中,3,5-二硝基水杨酸在与周围 NMP/乙醇/异丙醇/DMF 分子的相互作用中充当了路易斯酸。



























 京公网安备 11010802027423号
京公网安备 11010802027423号