当前位置:
X-MOL 学术
›
ACS Mater. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Developing Ideal Metalorganic Hydrides for Hydrogen Storage: From Theoretical Prediction to Rational Fabrication
ACS Materials Letters ( IF 11.4 ) Pub Date : 2021-08-24 , DOI: 10.1021/acsmaterialslett.1c00488 Zijun Jing 1, 2 , Qinqin Yuan 3 , Yang Yu 1 , Xiangtao Kong 4 , Khai Chen Tan 1, 2 , Jintao Wang 1, 2 , Qijun Pei 1 , Xue-Bin Wang 3 , Wei Zhou 5 , Hui Wu 5 , Anan Wu 6 , Teng He 1 , Ping Chen 1
ACS Materials Letters ( IF 11.4 ) Pub Date : 2021-08-24 , DOI: 10.1021/acsmaterialslett.1c00488 Zijun Jing 1, 2 , Qinqin Yuan 3 , Yang Yu 1 , Xiangtao Kong 4 , Khai Chen Tan 1, 2 , Jintao Wang 1, 2 , Qijun Pei 1 , Xue-Bin Wang 3 , Wei Zhou 5 , Hui Wu 5 , Anan Wu 6 , Teng He 1 , Ping Chen 1
Affiliation
|
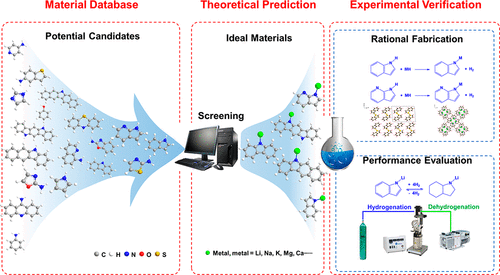
Materials for hydrogen storage have been extensively explored for a few decades. Thousands of materials have been synthesized and tested; however, few systems could meet the practical requirements. Metalorganic hydrides discovered recently offer new opportunities. It is, however, extremely time-consuming and inefficient to experimentally screen potential materials from a large variety of metal cations and organic anions. In the present study, we performed wide-ranging theoretical predictions and screened more than 90 metalorganic hydrides; 20 of them were identified with both high hydrogen capacities (≥5 wt %) and suitable thermodynamics (heat of H2 desorption: 25–35 kJ/mol-H2) that allow hydrogen uptake and release near ambient condition. We picked up four of them, i.e., Li/Na indolides and 7-azaindolides, for experimental validation. All the four candidates can be easily synthesized via the reactions between the metal hydrides and the corresponding organic precursors. Among them the structures of lithium indolide (P21/c (no. 14)) and sodium 7-azaindolide (P4̅21c (no. 114) were successfully resolved. Photoelectron spectroscopy and quantum chemical calculations confirmed that the charge density of the organic ring increased with the introduction of an alkali metal, thus optimizing their ΔHd for hydrogen storage. Importantly, we demonstrated experimentally that lithium indolide with a theoretical hydrogen capacity of 6.1 wt % has a heat of hydrogen absorption of ca. 33.7 kJ/mol-H2 which is in excellent agreement with the value (33.6 kJ/mol-H2) predicted theoretically. The partially reversible hydrogen release and uptake can be can be achieved at the temperature as low as 100 °C. These results illustrate the great potential of metalorganic hydrides for tackling the grand challenge of hydrogen storage and manifest the effectiveness of theoretical prediction in guiding materials fabrication.
中文翻译:

开发用于储氢的理想金属有机氢化物:从理论预测到合理制造
储氢材料已被广泛探索了几十年。数以千计的材料已被合成和测试;然而,很少有系统能够满足实际要求。最近发现的金属有机氢化物提供了新的机会。然而,从各种金属阳离子和有机阴离子中通过实验筛选潜在材料非常耗时且效率低下。在本研究中,我们进行了广泛的理论预测并筛选了 90 多种金属有机氢化物;其中 20 个被确定具有高氢容量(≥5 wt %)和合适的热力学(H 2解吸热:25–35 kJ/mol-H 2) 允许在接近环境条件下吸收和释放氢气。我们选取了其中的四种,即 Li/Na indolides 和 7-azaindolides,用于实验验证。所有四种候选物都可以通过金属氢化物与相应的有机前体之间的反应轻松合成。其中吲哚锂(P 2 1 / c (no. 14))和7-氮杂吲哚钠(P 4̅2 1 c (no. 114))的结构被成功解析。光电子能谱和量子化学计算证实了有机环随着碱金属的引入而增加,从而优化了它们的 Δ H d用于储氢。重要的是,我们通过实验证明了理论氢容量为 6.1 wt% 的吲哚锂的吸氢热约为。33.7 kJ/mol-H 2与理论预测值(33.6 kJ/mol-H 2)非常吻合。可在低至 100 °C 的温度下实现部分可逆的氢释放和吸收。这些结果说明了金属有机氢化物在应对储氢这一巨大挑战方面的巨大潜力,并证明了理论预测在指导材料制造方面的有效性。
更新日期:2021-09-06
中文翻译:

开发用于储氢的理想金属有机氢化物:从理论预测到合理制造
储氢材料已被广泛探索了几十年。数以千计的材料已被合成和测试;然而,很少有系统能够满足实际要求。最近发现的金属有机氢化物提供了新的机会。然而,从各种金属阳离子和有机阴离子中通过实验筛选潜在材料非常耗时且效率低下。在本研究中,我们进行了广泛的理论预测并筛选了 90 多种金属有机氢化物;其中 20 个被确定具有高氢容量(≥5 wt %)和合适的热力学(H 2解吸热:25–35 kJ/mol-H 2) 允许在接近环境条件下吸收和释放氢气。我们选取了其中的四种,即 Li/Na indolides 和 7-azaindolides,用于实验验证。所有四种候选物都可以通过金属氢化物与相应的有机前体之间的反应轻松合成。其中吲哚锂(P 2 1 / c (no. 14))和7-氮杂吲哚钠(P 4̅2 1 c (no. 114))的结构被成功解析。光电子能谱和量子化学计算证实了有机环随着碱金属的引入而增加,从而优化了它们的 Δ H d用于储氢。重要的是,我们通过实验证明了理论氢容量为 6.1 wt% 的吲哚锂的吸氢热约为。33.7 kJ/mol-H 2与理论预测值(33.6 kJ/mol-H 2)非常吻合。可在低至 100 °C 的温度下实现部分可逆的氢释放和吸收。这些结果说明了金属有机氢化物在应对储氢这一巨大挑战方面的巨大潜力,并证明了理论预测在指导材料制造方面的有效性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号