Journal of Physics and Chemistry of Solids ( IF 4 ) Pub Date : 2021-08-23 , DOI: 10.1016/j.jpcs.2021.110353 Theepakorn Sansenya 1 , Nataporn Masri 1 , Tammanoon Chankhanittha 1 , Teeradech Senasu 1 , Jirayus Piriyanon 1 , Siriboon Mukdasai 1 , Suwat Nanan 1
|
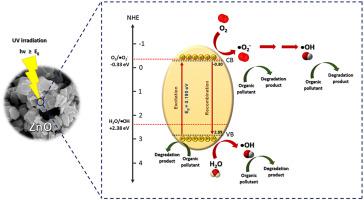
The ZnO photocatalysts were prepared by a hydrothermal method by using the Zn2+/OH− mole ratio of 1:5, 1:12, and 1:18 denoted as ZnO-1, ZnO-2, and ZnO-3, respectively. All prepared photocatalysts showed the hexagonal phase with the band gap energy of around 3.19 eV and the specific surface area of about 10.6 m2g-1. These photocatalysts remained stable even heating up to 1000 °C. Interestingly, the synthesized ZnO-1photocatalyst provided the lowest PL intensity among all prepared photocatalysts. This indicated the lowest electron-hole recombination rate at the interface. Therefore, this sample exhibited the highest photoactivity. The complete photodegradation of RR141, CR, and OFL pollutants after 20, 60 and 180 min of solar light irradiation, respectively, was obtained. The photodegradation of the pollutants correlated well with the first-order kinetics model. The corresponding rate constants of 0.1432, 0.0494 and 0.0304 min−1 toward degradation of RR141, CR and OFL, respectively, were reported. The stability of the ZnO-1 photocatalyst after three times of use was confirmed. The ZnO-1 photocatalyst still provided high performance even after the third cycle indicating its promising reusability. The photogenerated electron played a vital role in removal of the pollutants. The present research demonstrates a promising route for fabrication of ZnO photocatalyst with high efficiency for detoxification of organic pollutants in wastewater.
中文翻译:

水热合成氧化锌光催化剂用于阴离子偶氮染料和抗生素的解毒
ZnO 光催化剂是通过水热法制备的,使用 Zn 2+ /OH -摩尔比为 1:5、1:12 和 1:18,分别表示为 ZnO-1、ZnO-2 和 ZnO-3。所有制备的光催化剂均呈六方相,带隙能量约为 3.19 eV,比表面积约为 10.6 m 2 g -1. 这些光催化剂即使加热到 1000°C 也能保持稳定。有趣的是,合成的 ZnO-1 光催化剂在所有制备的光催化剂中提供了最低的 PL 强度。这表明界面处的电子-空穴复合率最低。因此,该样品表现出最高的光活性。分别在太阳光照射 20、60 和 180 分钟后获得了 RR141、CR 和 OFL 污染物的完全光降解。污染物的光降解与一级动力学模型有很好的相关性。相应的速率常数为 0.1432、0.0494 和 0.0304 min -1分别报道了 RR141、CR 和 OFL 的降解。ZnO-1 光催化剂在使用 3 次后的稳定性得到证实。即使在第三次循环后,ZnO-1 光催化剂仍能提供高性能,表明其具有良好的可重复使用性。光生电子对污染物的去除起到了至关重要的作用。目前的研究证明了一种有前途的制备氧化锌光催化剂的途径,该催化剂具有高效的废水中有机污染物的解毒能力。



























 京公网安备 11010802027423号
京公网安备 11010802027423号