当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Solubility of Ethanethiol in Water
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2021-08-23 , DOI: 10.1021/acs.jced.1c00358 Fang-Yuan Jou 1 , Alan E. Mather 1 , Kurt A. G. Schmidt 1
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2021-08-23 , DOI: 10.1021/acs.jced.1c00358 Fang-Yuan Jou 1 , Alan E. Mather 1 , Kurt A. G. Schmidt 1
Affiliation
|
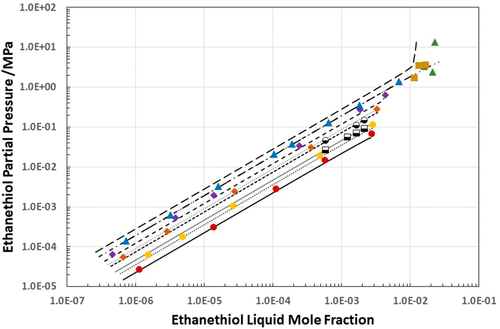
There are limited data in the open literature for the solubility of ethanethiol in water. A comprehensive literature review showed voids in the data set. The gaps in the literature data set were addressed with new experimental solubility measurements at temperatures in the range (298.15 to 413.15) K at pressures up to 1.75 MPa. The Peng-Robinson equation of state, with a temperature dependent binary interaction parameter, was used to correlate the new and existing data. The new solubility data were correlated to an average absolute percentage deviation of 1.3% in pressure and 8.1% in ethanethiol mole fraction. The VLLE phase boundary pressures of ethanethiol + water at (298.15, 313.15, 343.15, 373.15, and 413.15) K were also measured. Henry’s law constants, from the literature and those calculated from the Peng-Robinson equation of state, were correlated with a Clarke and Glew model over the (273.15 to 588.7) K temperature range. This model could correlate all of the Henry’s law constants to within an overall average absolute percentage deviation of 13.1%.
中文翻译:

乙硫醇在水中的溶解度
公开文献中关于乙硫醇在水中的溶解度的数据有限。综合文献综述显示数据集中存在空白。文献数据集中的空白通过在 (298.15 到 413.15) K 范围内的温度和高达 1.75 MPa 的压力下进行的新实验溶解度测量得到解决。Peng-Robinson 状态方程具有与温度相关的二元相互作用参数,用于关联新数据和现有数据。新的溶解度数据与 1.3% 的压力和 8.1% 的乙硫醇摩尔分数的平均绝对百分比偏差相关。还测量了乙硫醇 + 水在 (298.15、313.15、343.15、373.15 和 413.15) K 处的 VLLE 相边界压力。亨利定律常数,来自文献和根据彭-罗宾逊状态方程计算的常数,在(273.15 至 588.7)K 温度范围内与 Clarke 和 Glew 模型相关。该模型可以将所有亨利定律常数与 13.1% 的总体平均绝对百分比偏差相关联。
更新日期:2021-08-23
中文翻译:

乙硫醇在水中的溶解度
公开文献中关于乙硫醇在水中的溶解度的数据有限。综合文献综述显示数据集中存在空白。文献数据集中的空白通过在 (298.15 到 413.15) K 范围内的温度和高达 1.75 MPa 的压力下进行的新实验溶解度测量得到解决。Peng-Robinson 状态方程具有与温度相关的二元相互作用参数,用于关联新数据和现有数据。新的溶解度数据与 1.3% 的压力和 8.1% 的乙硫醇摩尔分数的平均绝对百分比偏差相关。还测量了乙硫醇 + 水在 (298.15、313.15、343.15、373.15 和 413.15) K 处的 VLLE 相边界压力。亨利定律常数,来自文献和根据彭-罗宾逊状态方程计算的常数,在(273.15 至 588.7)K 温度范围内与 Clarke 和 Glew 模型相关。该模型可以将所有亨利定律常数与 13.1% 的总体平均绝对百分比偏差相关联。



























 京公网安备 11010802027423号
京公网安备 11010802027423号