当前位置:
X-MOL 学术
›
J. Heterocycl. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Direct synthesis of 3,5-diaryl-1,2,4-oxadiazoles using 1-(2-oxo-2-arylethyl)pyridin-1-iums with benzamidines
Journal of Heterocyclic Chemistry ( IF 2.4 ) Pub Date : 2021-08-22 , DOI: 10.1002/jhet.4354 Yue Zhang 1 , Chengjun Wu 1 , Xinyi Wan 1 , Cunde Wang 1
Journal of Heterocyclic Chemistry ( IF 2.4 ) Pub Date : 2021-08-22 , DOI: 10.1002/jhet.4354 Yue Zhang 1 , Chengjun Wu 1 , Xinyi Wan 1 , Cunde Wang 1
Affiliation
|
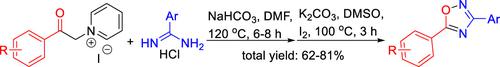
An efficient domino protocol for the synthesis of 1,2,4-oxadiazole derivatives from readily available 1-(2-oxo-2-arylethyl)pyridin-1-iums and amidine hydrochlorides was developed. In this practical approach, N-acyl amidine precursors were formed firstly via a simple nucleophilic substitution, without the purification of N-acylamidine intermediates, and the following intramolecularly dehydrative cyclization gave 1,2,4-oxadiazole derivatives in the presence of I2/K2CO3/DMSO, which exhibited excellent functional group tolerance and proceeded under simple experimental conditions.
中文翻译:

使用 1-(2-oxo-2-arylethyl)pyridin-1-iums 和苯甲脒直接合成 3,5-二芳基-1,2,4-恶二唑
开发了一种用于从容易获得的 1-(2-oxo-2-arylethyl)pyridin-1-iums 和脒盐酸盐合成 1,2,4-恶二唑衍生物的有效多米诺协议。在该实用的方法,Ñ酰基脒前体通过简单的亲核取代首先形成,未经纯化Ñ -acylamidine中间体和分子内下列脱水闭环中的我的存在下,得到1,2,4-恶二唑衍生物2 / K 2 CO 3 /DMSO 表现出优异的官能团耐受性并在简单的实验条件下进行。
更新日期:2021-08-22
中文翻译:

使用 1-(2-oxo-2-arylethyl)pyridin-1-iums 和苯甲脒直接合成 3,5-二芳基-1,2,4-恶二唑
开发了一种用于从容易获得的 1-(2-oxo-2-arylethyl)pyridin-1-iums 和脒盐酸盐合成 1,2,4-恶二唑衍生物的有效多米诺协议。在该实用的方法,Ñ酰基脒前体通过简单的亲核取代首先形成,未经纯化Ñ -acylamidine中间体和分子内下列脱水闭环中的我的存在下,得到1,2,4-恶二唑衍生物2 / K 2 CO 3 /DMSO 表现出优异的官能团耐受性并在简单的实验条件下进行。



























 京公网安备 11010802027423号
京公网安备 11010802027423号