当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Selective Neutral pH Inhibitor of Cathepsin B Designed Based on Cleavage Preferences at Cytosolic and Lysosomal pH Conditions
ACS Chemical Biology ( IF 4 ) Pub Date : 2021-08-20 , DOI: 10.1021/acschembio.1c00138 Michael C Yoon 1, 2 , Angelo Solania 3 , Zhenze Jiang 1 , Mitchell P Christy 4 , Sonia Podvin 1 , Charles Mosier 1 , Christopher B Lietz 1 , Gen Ito 1 , William H Gerwick 4 , Dennis W Wolan 3 , Gregory Hook 5 , Anthony J O'Donoghue 1 , Vivian Hook 1, 6
ACS Chemical Biology ( IF 4 ) Pub Date : 2021-08-20 , DOI: 10.1021/acschembio.1c00138 Michael C Yoon 1, 2 , Angelo Solania 3 , Zhenze Jiang 1 , Mitchell P Christy 4 , Sonia Podvin 1 , Charles Mosier 1 , Christopher B Lietz 1 , Gen Ito 1 , William H Gerwick 4 , Dennis W Wolan 3 , Gregory Hook 5 , Anthony J O'Donoghue 1 , Vivian Hook 1, 6
Affiliation
|
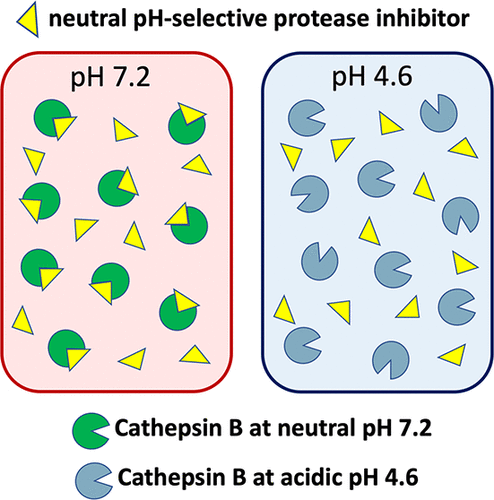
Cathepsin B is a cysteine protease that normally functions within acidic lysosomes for protein degradation, but in numerous human diseases, cathepsin B translocates to the cytosol having neutral pH where the enzyme activates inflammation and cell death. Cathepsin B is active at both the neutral pH 7.2 of the cytosol and the acidic pH 4.6 within lysosomes. We evaluated the hypothesis that cathepsin B may possess pH-dependent cleavage preferences that can be utilized for design of a selective neutral pH inhibitor by (1) analysis of differential cathepsin B cleavage profiles at neutral pH compared to acidic pH using multiplex substrate profiling by mass spectrometry (MSP-MS), (2) design of pH-selective peptide–7-amino-4-methylcoumarin (AMC) substrates, and (3) design and validation of Z-Arg-Lys-acyloxymethyl ketone (AOMK) as a selective neutral pH inhibitor. Cathepsin B displayed preferences for cleaving peptides with Arg in the P2 position at pH 7.2 and Glu in the P2 position at pH 4.6, represented by its primary dipeptidyl carboxypeptidase and modest endopeptidase activity. These properties led to design of the substrate Z-Arg-Lys–AMC having neutral pH selectivity, and its modification with the AOMK warhead to result in the inhibitor Z-Arg-Lys–AOMK. This irreversible inhibitor displays nanomolar potency with 100-fold selectivity for inhibition of cathepsin B at pH 7.2 compared to pH 4.6, shows specificity for cathepsin B over other cysteine cathepsins, and is cell permeable and inhibits intracellular cathepsin B. These findings demonstrate that cathepsin B possesses pH-dependent cleavage properties that can lead to development of a potent, neutral pH inhibitor of this enzyme.
中文翻译:

基于细胞溶质和溶酶体 pH 条件下的切割偏好设计的组织蛋白酶 B 选择性中性 pH 抑制剂
组织蛋白酶 B 是一种半胱氨酸蛋白酶,通常在酸性溶酶体内起作用以降解蛋白质,但在许多人类疾病中,组织蛋白酶 B 易位至具有中性 pH 值的胞质溶胶,在此酶激活炎症和细胞死亡。组织蛋白酶 B 在胞质溶胶的中性 pH 值 7.2 和溶酶体内的酸性 pH 值 4.6 下均具有活性。我们评估了组织蛋白酶 B 可能具有 pH 依赖性切割偏好的假设,该偏好可用于设计选择性中性 pH 抑制剂,方法是 (1) 使用多重底物质量分析,与酸性 pH 相比,在中性 pH 下分析不同的组织蛋白酶 B 切割谱光谱法(MSP-MS),(2)pH选择性肽-7-氨基-4-甲基香豆素(AMC)底物的设计,(3) Z-Arg-Lys-酰氧基甲基酮 (AOMK) 作为选择性中性 pH 抑制剂的设计和验证。组织蛋白酶 B 表现出偏好在 pH 7.2 时在 P2 位置使用 Arg 在 pH 4.6 时在 P2 位置切割肽,这表现为其主要的二肽基羧肽酶和适度的内肽酶活性。这些特性导致设计了具有中性 pH 选择性的底物 Z-Arg-Lys-AMC,并用 AOMK 弹头对其进行改性以产生抑制剂 Z-Arg-Lys-AOMK。与 pH 4.6 相比,这种不可逆的抑制剂在 pH 7.2 时对组织蛋白酶 B 的抑制选择性是纳摩尔浓度的 100 倍,对组织蛋白酶 B 的特异性优于其他半胱氨酸组织蛋白酶,并且具有细胞渗透性并抑制细胞内组织蛋白酶 B。
更新日期:2021-09-17
中文翻译:

基于细胞溶质和溶酶体 pH 条件下的切割偏好设计的组织蛋白酶 B 选择性中性 pH 抑制剂
组织蛋白酶 B 是一种半胱氨酸蛋白酶,通常在酸性溶酶体内起作用以降解蛋白质,但在许多人类疾病中,组织蛋白酶 B 易位至具有中性 pH 值的胞质溶胶,在此酶激活炎症和细胞死亡。组织蛋白酶 B 在胞质溶胶的中性 pH 值 7.2 和溶酶体内的酸性 pH 值 4.6 下均具有活性。我们评估了组织蛋白酶 B 可能具有 pH 依赖性切割偏好的假设,该偏好可用于设计选择性中性 pH 抑制剂,方法是 (1) 使用多重底物质量分析,与酸性 pH 相比,在中性 pH 下分析不同的组织蛋白酶 B 切割谱光谱法(MSP-MS),(2)pH选择性肽-7-氨基-4-甲基香豆素(AMC)底物的设计,(3) Z-Arg-Lys-酰氧基甲基酮 (AOMK) 作为选择性中性 pH 抑制剂的设计和验证。组织蛋白酶 B 表现出偏好在 pH 7.2 时在 P2 位置使用 Arg 在 pH 4.6 时在 P2 位置切割肽,这表现为其主要的二肽基羧肽酶和适度的内肽酶活性。这些特性导致设计了具有中性 pH 选择性的底物 Z-Arg-Lys-AMC,并用 AOMK 弹头对其进行改性以产生抑制剂 Z-Arg-Lys-AOMK。与 pH 4.6 相比,这种不可逆的抑制剂在 pH 7.2 时对组织蛋白酶 B 的抑制选择性是纳摩尔浓度的 100 倍,对组织蛋白酶 B 的特异性优于其他半胱氨酸组织蛋白酶,并且具有细胞渗透性并抑制细胞内组织蛋白酶 B。



























 京公网安备 11010802027423号
京公网安备 11010802027423号