当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
OFF-State-Specific Inhibition of the Proprotein Convertase Furin
ACS Chemical Biology ( IF 4 ) Pub Date : 2021-08-20 , DOI: 10.1021/acschembio.1c00411 Sven O Dahms 1 , Tanja Haider 1 , Gerhard Klebe 2 , Torsten Steinmetzer 2 , Hans Brandstetter 1
ACS Chemical Biology ( IF 4 ) Pub Date : 2021-08-20 , DOI: 10.1021/acschembio.1c00411 Sven O Dahms 1 , Tanja Haider 1 , Gerhard Klebe 2 , Torsten Steinmetzer 2 , Hans Brandstetter 1
Affiliation
|
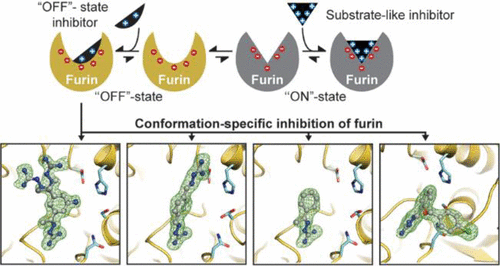
The pro-protein convertase furin is a highly specific serine protease involved in the proteolytic maturation of many proteins in the secretory pathway. It also activates surface proteins of many viruses including the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Furin inhibitors effectively suppress viral replication and thus are promising antiviral therapeutics with broad application potential. Polybasic substrate-like ligands typically trigger conformational changes shifting furin’s active site cleft from the OFF-state to the ON-state. Here, we solved the X-ray structures of furin in complex with four different arginine mimetic compounds with reduced basicity. These guanylhydrazone-based inhibitor complexes showed for the first time an active site-directed binding mode to furin’s OFF-state conformation. The compounds undergo unique interactions within the S1 pocket, largely different compared to substrate-like ligands. A second binding site was identified at the S4/S5 pocket of furin. Crystallography-based titration experiments confirmed the S1 site as the primary binding pocket. We also tested the proprotein convertases PC5/6 and PC7 for inhibition by guanylhydrazones and found an up to 7-fold lower potency for PC7. Interestingly, the observed differences in the Ki values correlated with the sequence conservation of the PCs at the allosteric sodium binding site. Therefore, OFF-state-specific targeting of furin can serve as a valuable strategy for structure-based development of PC-selective small-molecule inhibitors.
中文翻译:

前蛋白转化酶弗林蛋白酶的关闭状态特异性抑制
前蛋白转化酶弗林蛋白酶是一种高度特异性的丝氨酸蛋白酶,参与分泌途径中许多蛋白质的蛋白水解成熟。它还可以激活许多病毒的表面蛋白,包括严重急性呼吸系统综合症冠状病毒 2 (SARS-CoV-2)。弗林蛋白酶抑制剂有效抑制病毒复制,因此是具有广泛应用潜力的有前途的抗病毒疗法。多元底物样配体通常会触发构象变化,将弗林蛋白酶的活性位点裂缝从关闭状态转变为开启状态。在这里,我们解决了与四种不同的碱性降低的精氨酸模拟化合物复合的弗林蛋白酶的 X 射线结构。这些基于脒腙的抑制剂复合物首次显示出与弗林蛋白酶 OFF 状态构象的活性定点结合模式。这些化合物在 S1 口袋内经历独特的相互作用,与底物样配体相比有很大不同。在弗林蛋白酶的 S4/S5 口袋处鉴定了第二个结合位点。基于晶体学的滴定实验证实 S1 位点是主要的结合口袋。我们还测试了前蛋白转化酶 PC5/6 和 PC7 对脒腙的抑制作用,发现 PC7 的效力降低了 7 倍。有趣的是,观察到的差异 我们还测试了前蛋白转化酶 PC5/6 和 PC7 对脒腙的抑制作用,发现 PC7 的效力降低了 7 倍。有趣的是,观察到的差异 我们还测试了前蛋白转化酶 PC5/6 和 PC7 对脒腙的抑制作用,发现 PC7 的效力降低了 7 倍。有趣的是,观察到的差异K i值与变构钠结合位点处 PC 的序列保守性相关。因此,弗林蛋白酶的 OFF 状态特异性靶向可以作为基于结构开发 PC 选择性小分子抑制剂的有价值的策略。
更新日期:2021-09-17
中文翻译:

前蛋白转化酶弗林蛋白酶的关闭状态特异性抑制
前蛋白转化酶弗林蛋白酶是一种高度特异性的丝氨酸蛋白酶,参与分泌途径中许多蛋白质的蛋白水解成熟。它还可以激活许多病毒的表面蛋白,包括严重急性呼吸系统综合症冠状病毒 2 (SARS-CoV-2)。弗林蛋白酶抑制剂有效抑制病毒复制,因此是具有广泛应用潜力的有前途的抗病毒疗法。多元底物样配体通常会触发构象变化,将弗林蛋白酶的活性位点裂缝从关闭状态转变为开启状态。在这里,我们解决了与四种不同的碱性降低的精氨酸模拟化合物复合的弗林蛋白酶的 X 射线结构。这些基于脒腙的抑制剂复合物首次显示出与弗林蛋白酶 OFF 状态构象的活性定点结合模式。这些化合物在 S1 口袋内经历独特的相互作用,与底物样配体相比有很大不同。在弗林蛋白酶的 S4/S5 口袋处鉴定了第二个结合位点。基于晶体学的滴定实验证实 S1 位点是主要的结合口袋。我们还测试了前蛋白转化酶 PC5/6 和 PC7 对脒腙的抑制作用,发现 PC7 的效力降低了 7 倍。有趣的是,观察到的差异 我们还测试了前蛋白转化酶 PC5/6 和 PC7 对脒腙的抑制作用,发现 PC7 的效力降低了 7 倍。有趣的是,观察到的差异 我们还测试了前蛋白转化酶 PC5/6 和 PC7 对脒腙的抑制作用,发现 PC7 的效力降低了 7 倍。有趣的是,观察到的差异K i值与变构钠结合位点处 PC 的序列保守性相关。因此,弗林蛋白酶的 OFF 状态特异性靶向可以作为基于结构开发 PC 选择性小分子抑制剂的有价值的策略。


























 京公网安备 11010802027423号
京公网安备 11010802027423号