Energy Storage Materials ( IF 20.4 ) Pub Date : 2021-08-21 , DOI: 10.1016/j.ensm.2021.08.024 Sojung Koo 1 , Jaewoon Lee 1 , Jinwoo Lee 1 , Sangho Yoon 1 , Duho Kim 1
|
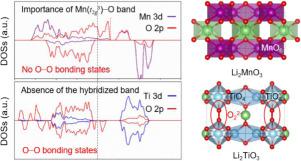
Although nearly all oxygen redox reaction (ORR) research has been carried out based on Mn4+-containing layered oxides (Li[Li1−x−yMnxMy]O2) in Li-ion positive electrodes, the roles of Mn(t2g3)−O hybridized band in correlation with ORRs have yet to be understood. Considering the competition of covalent and ionic M−O bond features, the two-type Li2MO3 cathode models (i.e., 3d3-Li2MnO3 and 3d0-Li2TiO3) operated by the pure ORRs were investigated using first-principles calculations to unlock veiled critical factors in activating ORRs, and enabling their reversibility upon charging. First, the thermodynamic formation energies showed that the oxygen instability induced by Li+-extraction is much larger for Li2TiO3 than that for Li2MnO3. Second, the [[EQUATION]]-type Mn(t2g3)−O hybridized band in the low energy state for the Mn oxide broadened the O 2p-bandwidth, resulting in weakening the chemical hardness of Mn−O in comparison to that of Ti−O. Third, the cooperative inter- and intra-layer O−O dimerizations (< 1.5 Å) were formed with the O decoordination from the TiO6- containing layer in the highly delithiated phase, while the short O−O dimers were present within the Mn intralayer for Li2MnO3. From this theoretical understanding, we have concluded that the presence of rigid Mn(t2g3)−O band upon charging plays a significant role in easily triggering the oxygen redox, and in suppressing the formation of very short O−O dimers leading to O2 release, which are because of the non-overlap between the bonding states of O−O and the Mn(t2g3)−O state. The revealed importance of Mn(t2g3)−O band provides an exciting direction for designing ORutilizing high energy density cathodes for lithium-ion batteries.
中文翻译:

金属-氧键对锂过量层状氧化物稳定氧-氧化还原反应的重要性
尽管几乎所有的氧氧化还原反应 (ORR) 研究都是基于锂离子正极中含 Mn4+ 的层状氧化物 (Li[Li1−x−yMnxMy]O2) 进行的,但 Mn(t2g3)−O 杂化带的作用与 ORR 的相关性尚待了解。考虑到共价键和离子 M−O 键特征的竞争,使用第一性原理计算研究了由纯 ORR 操作的两种类型的 Li2MO3 阴极模型(即 3d3-Li2MnO3 和 3d0-Li2TiO3),以解开隐藏的激活关键因素ORR,并在充电时使其可逆。首先,热力学形成能表明,Li2TiO3 比 Li2MnO3 大得多。其次,[[方程式]]型Mn(t2g3)-O杂化带在低能态Mn氧化物加宽了O 2p-带宽,导致Mn-O与Ti-O相比的化学硬度减弱. 第三,协同的层间和层内 O-O 二聚体(< 1.5 Å)是由高度脱锂相中含 TiO6 层的 O 脱配形成的,而短的 O-O 二聚体存在于 Mn 层内对于 Li2MnO3。根据这一理论理解,我们得出结论,充电时刚性 Mn(t2g3)-O 带的存在在容易触发氧氧化还原和抑制导致 O2 释放的非常短的 O-O 二聚体的形成方面起着重要作用,这是因为 O-O 和 Mn(t2g3)-O 状态之间的键合状态不重叠。



























 京公网安备 11010802027423号
京公网安备 11010802027423号