Journal of Microbiological Methods ( IF 2.2 ) Pub Date : 2021-08-21 , DOI: 10.1016/j.mimet.2021.106312 Francielle Regina Silva Dias 1 , Felipe Rebello Lourenço 1
|
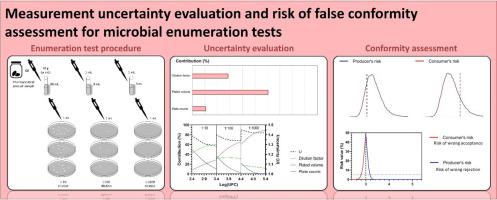
Microbial enumeration tests are widely used to assess the microbiological quality of non-sterile pharmaceutical products. Despite of all efforts to guarantee the reliability of microbial enumeration tests, there will always be an uncertainty associated with the measured values, which can lead to false conformity/non-conformity decisions. In this work, we evaluated the measurement uncertainty using a bottom-up approach and estimate the consumer's or producer's risk due to the measurement uncertainty. Three main sources of uncertainty were identified and quantified: dilution factor, plated volume, and microbial plate counts. The contribution of these sources of uncertainty depends on the measured value of microbial load in pharmaceutical products. The contribution of dilution factor and plated volume uncertainties increase with an increase of measured value, while the contribution of microbial plate count uncertainty decreases with an increase of measured value. The overall uncertainty values were expressed as uncertainty factors, which provide an asymmetric 95% level confidence level of microbial load in pharmaceutical products. In addition, the risk of false conformity/non-conformity decisions due to measurement uncertainty was assess using Monte Carlo method. When the measured value is close to the upper specification limit and/or the measurement uncertainty is large, the risk of false conformity/non-conformity decisions may be significantly high. Thus, we conclude that the use of uncertainty factor in the conformity/non-conformity assessment is important to guarantee the reliability of microbial enumeration test results and to support decision-making.
中文翻译:

微生物计数测试的测量不确定度评估和错误合格评定的风险
微生物计数测试广泛用于评估非无菌药品的微生物质量。尽管尽了所有努力来保证微生物计数测试的可靠性,但始终存在与测量值相关的不确定性,这可能导致错误的合格/不合格决定。在这项工作中,我们使用自下而上的方法评估了测量不确定性,并估计了由于测量不确定性导致的消费者或生产者的风险。确定并量化了三个主要的不确定性来源:稀释因子、镀层体积和微生物板计数。这些不确定性来源的贡献取决于药品中微生物负荷的测量值。稀释因子和平板体积不确定度的贡献随着测量值的增加而增加,而微生物平板计数不确定度的贡献随着测量值的增加而减小。总体不确定性值表示为不确定性因子,它提供了医药产品中微生物负荷的不对称 95% 置信水平。此外,使用蒙特卡罗方法评估了由于测量不确定性导致的错误合格/不合格决策的风险。当测量值接近规格上限和/或测量不确定度很大时,错误的合格/不合格决定的风险可能非常高。因此,



























 京公网安备 11010802027423号
京公网安备 11010802027423号