Cell Reports Physical Science ( IF 8.9 ) Pub Date : 2021-08-20 , DOI: 10.1016/j.xcrp.2021.100545 Varun Mohan 1 , Biswanath Dutta 2 , Roma Ripani 2 , Prashant K. Jain 2, 3, 4
|
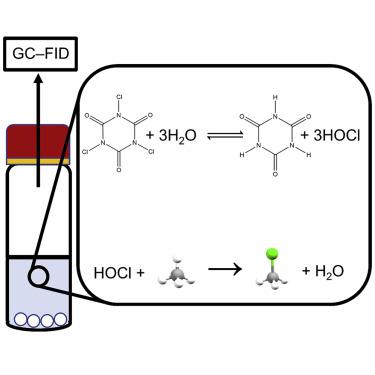
The chlorination of methane presents a route for the upgrading of natural gas to value-added products. However, due to the low reactivity of methane, existing chlorination processes require the use of elevated temperatures and catalysts. Here, we report a simple process for the chlorination of methane at near-ambient temperatures using minimal reagents and no catalysts or external sources of energy. The reaction is carried out in an aqueous medium with trichloroisocyanuric acid as a chlorinating agent. The dissolution of trichloroisocyanuric acid in water leads to the sustained and buffered release of hypochlorous acid, which triggers the chlorination of methane by a free-radical mechanism. The process is also general to other alkanes, as shown by a similar chlorination of ethane. Further developments are required for this process to be deployed as a practical method of chloromethane production.
中文翻译:

室温无催化剂甲烷氯化
甲烷氯化提供了一条将天然气升级为增值产品的途径。然而,由于甲烷的低反应性,现有的氯化工艺需要使用高温和催化剂。在这里,我们报告了一种在接近环境温度下使用最少的试剂和没有催化剂或外部能源来氯化甲烷的简单过程。该反应在水性介质中以三氯异氰脲酸作为氯化剂进行。三氯异氰尿酸在水中的溶解导致次氯酸的持续和缓冲释放,从而通过自由基机制触发甲烷的氯化。该过程也适用于其他烷烃,如乙烷的类似氯化所示。



























 京公网安备 11010802027423号
京公网安备 11010802027423号