当前位置:
X-MOL 学术
›
Pept. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
De novo design of metal-binding cleft in a Trp-Trp stapled thermostable β-hairpin peptide
Peptide Science ( IF 2.4 ) Pub Date : 2021-08-18 , DOI: 10.1002/pep2.24240 Muralikrishna Lella 1 , Radhakrishnan Mahalakshmi 1
Peptide Science ( IF 2.4 ) Pub Date : 2021-08-18 , DOI: 10.1002/pep2.24240 Muralikrishna Lella 1 , Radhakrishnan Mahalakshmi 1
Affiliation
|
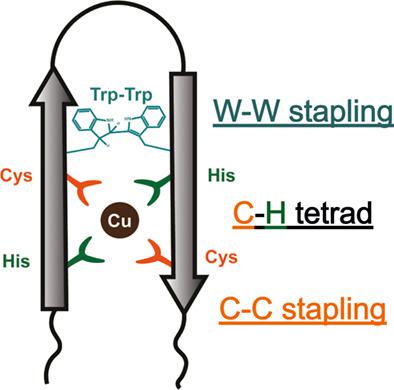
Metals are important molecules in protein biochemistry, as they are involved in various essential biochemical processes. Inspired by the coordination chemistry of metal-binding proteins, and to improvise the stability of recognition motifs, here we present the design of hyperstable stapled β-hairpin with a defined metal-binding cleft. We achieved this by establishing Trp-Trp covalent cross-linking in a long-chain 16-residue β-hairpin scaffold, and incorporating a stereospecific Cys2-His2 tetrad as the metal-binding cleft. This water-soluble peptide showed broad metal-binding properties, with Cu2+ specificity. Importance of the Cys-His tetrad in metal ion selectivity was established with Asp/Glu substitutions. We propose that such predefined hyperstable β-hairpins with recognition motifs are useful versatile tools for developing peptide-based catalysts, and in biomarker design.
中文翻译:

Trp-Trp 钉合的热稳定 β-发夹肽中金属结合裂缝的从头设计
金属是蛋白质生物化学中的重要分子,因为它们参与各种基本的生化过程。受金属结合蛋白的配位化学的启发,并为了提高识别基序的稳定性,我们在这里提出了具有明确金属结合裂缝的超稳定钉合 β-发夹的设计。我们通过在长链 16 个残基 β-发夹支架中建立 Trp-Trp 共价交联并结合立体特异性 Cys 2 -His 2四分体作为金属结合裂缝来实现这一点。这种水溶性肽显示出广泛的金属结合特性,与 Cu 2+特异性。Cys-His 四联体在金属离子选择性中的重要性是通过 Asp/Glu 取代确定的。我们建议这种具有识别基序的预定义超稳定 β-发夹是用于开发基于肽的催化剂和生物标志物设计的有用的通用工具。
更新日期:2021-08-18
中文翻译:

Trp-Trp 钉合的热稳定 β-发夹肽中金属结合裂缝的从头设计
金属是蛋白质生物化学中的重要分子,因为它们参与各种基本的生化过程。受金属结合蛋白的配位化学的启发,并为了提高识别基序的稳定性,我们在这里提出了具有明确金属结合裂缝的超稳定钉合 β-发夹的设计。我们通过在长链 16 个残基 β-发夹支架中建立 Trp-Trp 共价交联并结合立体特异性 Cys 2 -His 2四分体作为金属结合裂缝来实现这一点。这种水溶性肽显示出广泛的金属结合特性,与 Cu 2+特异性。Cys-His 四联体在金属离子选择性中的重要性是通过 Asp/Glu 取代确定的。我们建议这种具有识别基序的预定义超稳定 β-发夹是用于开发基于肽的催化剂和生物标志物设计的有用的通用工具。

























 京公网安备 11010802027423号
京公网安备 11010802027423号