当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Iron-Catalyzed Alkyne Carboamination via an Isolable Iron Imide Complex
Organometallics ( IF 2.8 ) Pub Date : 2021-08-18 , DOI: 10.1021/acs.organomet.1c00454 Corey A. Richards 1 , Nigam P. Rath 2 , Jamie M. Neely 1
Organometallics ( IF 2.8 ) Pub Date : 2021-08-18 , DOI: 10.1021/acs.organomet.1c00454 Corey A. Richards 1 , Nigam P. Rath 2 , Jamie M. Neely 1
Affiliation
|
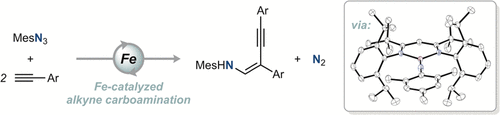
Transition metal imide-mediated C–N bond formation is a powerful strategy for the introduction of nitrogen into organic compounds. We have discovered that the reaction of N-mesityl(β-diketiminato)iron imide complex tBuLFeNMes (tBuL = 3,5-bis(2,6-diisopropylphenylimino)-2,2,6,6-tetramethylheptyl and Mes = 2,4,6-trimethylphenyl) with a terminal alkyne substrate gives a β-alkynyl enamine product by a novel alkyne carboamination process. Stoichiometric experiments revealed a catalyst deactivation pathway involving generation of the acetylide complex, tBuLFeCCPh, and mesityl amine (MesNH2) from the acetylene complex, tBuLFe(HCCPh), and mesityl azide (MesN3). This reactivity is suppressed in the presence of coordinating additive 4-tert-butylpyridine (tBuPy), likely through formation of the four-coordinate complex tBuLFe(HCCPh)(tBuPy). These insights were instrumental in identifying reaction conditions that allow for turnover of the iron catalyst.
中文翻译:

通过可分离的亚铁配合物进行铁催化的炔烃碳胺化
过渡金属酰亚胺介导的 C-N 键形成是将氮引入有机化合物的有效策略。我们发现N -mesityl(β-diketiminato)iron imide 络合物t Bu LFeNMes ( t Bu L = 3,5-bis(2,6-diisopropylphenylimino)-2,2,6,6-tetramethylheptyl 和 Mes = 2,4,6-三甲基苯基)与末端炔烃底物通过新型炔烃碳胺化过程产生 β-炔基烯胺产物。化学计量的实验揭示催化剂失活途径涉及产生乙炔化复杂的,吨卜LFeCCPh,和三甲苯基胺(MesNH 2)从乙炔复杂,吨卜LFe(HCCPh) 和甲基叠氮化物 (MesN 3 )。这种反应性在配位添加剂 4-叔丁基吡啶 ( t BuPy)的存在下受到抑制,可能是通过形成四配位配合物t Bu LFe(HCCPh)( t BuPy)。这些见解有助于确定允许铁催化剂周转的反应条件。
更新日期:2021-09-13
中文翻译:

通过可分离的亚铁配合物进行铁催化的炔烃碳胺化
过渡金属酰亚胺介导的 C-N 键形成是将氮引入有机化合物的有效策略。我们发现N -mesityl(β-diketiminato)iron imide 络合物t Bu LFeNMes ( t Bu L = 3,5-bis(2,6-diisopropylphenylimino)-2,2,6,6-tetramethylheptyl 和 Mes = 2,4,6-三甲基苯基)与末端炔烃底物通过新型炔烃碳胺化过程产生 β-炔基烯胺产物。化学计量的实验揭示催化剂失活途径涉及产生乙炔化复杂的,吨卜LFeCCPh,和三甲苯基胺(MesNH 2)从乙炔复杂,吨卜LFe(HCCPh) 和甲基叠氮化物 (MesN 3 )。这种反应性在配位添加剂 4-叔丁基吡啶 ( t BuPy)的存在下受到抑制,可能是通过形成四配位配合物t Bu LFe(HCCPh)( t BuPy)。这些见解有助于确定允许铁催化剂周转的反应条件。



























 京公网安备 11010802027423号
京公网安备 11010802027423号