当前位置:
X-MOL 学术
›
Biomater. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Surface engineering of oncolytic adenovirus for a combination of immune checkpoint blockade and virotherapy
Biomaterials Science ( IF 6.6 ) Pub Date : 2021-08-02 , DOI: 10.1039/d1bm00928a Peng Lv 1 , Xiaomei Chen 1 , Shiying Fu 1 , En Ren 1 , Chao Liu 1 , Xuan Liu 1 , Lai Jiang 1 , Yun Zeng 2 , Xiaoyong Wang 1, 3 , Gang Liu 1
Biomaterials Science ( IF 6.6 ) Pub Date : 2021-08-02 , DOI: 10.1039/d1bm00928a Peng Lv 1 , Xiaomei Chen 1 , Shiying Fu 1 , En Ren 1 , Chao Liu 1 , Xuan Liu 1 , Lai Jiang 1 , Yun Zeng 2 , Xiaoyong Wang 1, 3 , Gang Liu 1
Affiliation
|
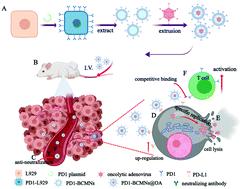
Advances in the development of modern cancer immunotherapy and immune checkpoint inhibitors have dramatically changed the landscape of cancer treatment. However, most cancer patients are refractory to immune checkpoint inhibitors because of low lymphocytic tumor infiltration and PD-L1 expression. Evidence suggests that viral oncolysis and immune checkpoint inhibitors have a synergistic effect that can improve the response to immune checkpoint inhibitors. In this study, we developed bioengineered cell membrane nanovesicles (PD1-BCMNs) with programmed cell death protein 1 (PD-1) to harbor oncolytic adenovirus (OA) and achieve a combination of immune checkpoint blockade and oncolytic virotherapy in one particle for cancer treatment. PD1-BCMNs could specifically deliver OA to tumor tissue; the infectivity and replication ability of the OA was preserved in the presence of neutralizing antibodies in vitro and in vivo. Selective oncolytic effects with oncolytic adenovirus led to an up-regulated expression of PD-L1 in the tumor microenvironment, turning immunologically ‘cold’ tumors into immunologically ‘hot’ tumors, presenting more targets for further enhanced target delivery. Notably, PD1-BCMNs@OA could effectively activate tumor-infiltrating T cells and elicit a strong anti-tumor immune response. Thus, PD1-BCMNs@OA may provide a clinical basis for combining oncolytic virotherapy with checkpoint inhibitors, enhancing the oncolytic adenovirus targeted delivery and significantly enhancing T cell immune responses, resulting in a stronger antitumor immunity response.
中文翻译:

用于免疫检查点阻断和病毒治疗相结合的溶瘤腺病毒的表面工程
现代癌症免疫疗法和免疫检查点抑制剂的发展进步极大地改变了癌症治疗的格局。然而,由于低淋巴细胞肿瘤浸润和 PD-L1 表达,大多数癌症患者对免疫检查点抑制剂无效。有证据表明,病毒溶瘤和免疫检查点抑制剂具有协同作用,可以提高对免疫检查点抑制剂的反应。在这项研究中,我们开发了具有程序性细胞死亡蛋白 1 (PD-1) 的生物工程细胞膜纳米囊泡 (PD1-BCMNs) 以容纳溶瘤腺病毒 (OA),并在一个粒子中实现免疫检查点阻断和溶瘤病毒疗法的组合用于癌症治疗. PD1-BCMNs 可以特异性地将 OA 递送至肿瘤组织;体外和体内。溶瘤腺病毒的选择性溶瘤作用导致肿瘤微环境中 PD-L1 的表达上调,将免疫“冷”肿瘤转变为免疫“热”肿瘤,为进一步增强靶点传递提供更多靶点。值得注意的是,PD1-BCMNs@OA 可以有效激活肿瘤浸润性 T 细胞并引发强烈的抗肿瘤免疫反应。因此,PD1-BCMNs@OA可能为溶瘤病毒疗法与检查点抑制剂相结合,增强溶瘤腺病毒靶向递送和显着增强T细胞免疫反应,从而产生更强的抗肿瘤免疫反应提供临床基础。
更新日期:2021-08-19
中文翻译:

用于免疫检查点阻断和病毒治疗相结合的溶瘤腺病毒的表面工程
现代癌症免疫疗法和免疫检查点抑制剂的发展进步极大地改变了癌症治疗的格局。然而,由于低淋巴细胞肿瘤浸润和 PD-L1 表达,大多数癌症患者对免疫检查点抑制剂无效。有证据表明,病毒溶瘤和免疫检查点抑制剂具有协同作用,可以提高对免疫检查点抑制剂的反应。在这项研究中,我们开发了具有程序性细胞死亡蛋白 1 (PD-1) 的生物工程细胞膜纳米囊泡 (PD1-BCMNs) 以容纳溶瘤腺病毒 (OA),并在一个粒子中实现免疫检查点阻断和溶瘤病毒疗法的组合用于癌症治疗. PD1-BCMNs 可以特异性地将 OA 递送至肿瘤组织;体外和体内。溶瘤腺病毒的选择性溶瘤作用导致肿瘤微环境中 PD-L1 的表达上调,将免疫“冷”肿瘤转变为免疫“热”肿瘤,为进一步增强靶点传递提供更多靶点。值得注意的是,PD1-BCMNs@OA 可以有效激活肿瘤浸润性 T 细胞并引发强烈的抗肿瘤免疫反应。因此,PD1-BCMNs@OA可能为溶瘤病毒疗法与检查点抑制剂相结合,增强溶瘤腺病毒靶向递送和显着增强T细胞免疫反应,从而产生更强的抗肿瘤免疫反应提供临床基础。



























 京公网安备 11010802027423号
京公网安备 11010802027423号