当前位置:
X-MOL 学术
›
Chem. Biodivers.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis, Characterization, and Biological Study of 3-Trifluoromethylpyrazole Tethered Chalcone-Pyrrole and Pyrazoline-Pyrrole Derivatives
Chemistry & Biodiversity ( IF 2.9 ) Pub Date : 2021-08-19 , DOI: 10.1002/cbdv.202100504 Nishant Kisan Rasal 1 , Rahul Bhaskar Sonawane 1 , Sangeeta Vijay Jagtap 1
Chemistry & Biodiversity ( IF 2.9 ) Pub Date : 2021-08-19 , DOI: 10.1002/cbdv.202100504 Nishant Kisan Rasal 1 , Rahul Bhaskar Sonawane 1 , Sangeeta Vijay Jagtap 1
Affiliation
|
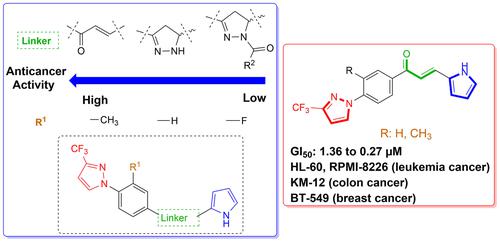
The present study illustrates the design and synthesis of new series of 3-trifluoromethylpyrazole tethered chalcone-pyrrole and pyrazoline-pyrrole derivatives. All compounds were further screened for in vitro cytostatic activities on full NCI 60 cancer cell lines at National Cancer Institute, USA. Compounds (2E)-3-(1H-pyrrol-2-yl)-1-{4-[3-(trifluoromethyl)-1H-pyrazol-1-yl]phenyl}prop-2-en-1-one (5a) and (2E)-1-{3-methyl-4-[3-(trifluoromethyl)-1H-pyrazol-1-yl]phenyl}-3-(1H-pyrrol-2-yl)prop-2-en-1-one (5c) displayed significant antiproliferative activity (Growth Percentage: −77.10 and −92.13, respectively at 10 μM concentration) against the UO-31 cell lines from renal cancer and were further selected for assay at 10-fold dilutions of five different concentrations (10−4 to 10−8 M). Both compounds 5a and 5c exhibited promising antiproliferative activity (GI50: 1.36 to 0.27 μM) against leukemia cancer cell lines HL-60 and RPMI-8226, colon cancer cell lines KM-12; breast cancer cell lines BT-549. Moreover, both compounds 5a and 5c were found to be non-cytotoxic (LC50>100) against HL-60, RPMI-8226, and KM-12 cell lines. Remarkably, GI50 values of compounds 5a and 5c were identified as more promising than sunitinib against most cancer cell lines. In silico study of compounds 5a and 5c exemplified the desired ADME properties for drug-likeness as well as tighter interactions with VEGFR-2. Hence, compounds 5a and 5c would be good cytotoxic agents after further clinical study.
中文翻译:

3-三氟甲基吡唑系链查尔酮-吡咯和吡唑啉-吡咯衍生物的合成、表征和生物学研究
本研究说明了新系列 3-三氟甲基吡唑系链查尔酮-吡咯和吡唑啉-吡咯衍生物的设计和合成。在美国国家癌症研究所进一步筛选所有化合物对完整 NCI 60 癌细胞系的体外细胞抑制活性。化合物( 2E )-3-( 1H-吡咯-2-基)-1-{4-[3-(三氟甲基) -1H-吡唑-1-基]苯基}prop-2-en-1-一( 5a )和( 2E )-1-{3-甲基-4-[3-(三氟甲基) -1H-吡唑-1-基]苯基}-3-( 1H-吡咯-2-基) prop-2-en-1-one ( 5c)显示显著的抗增殖活性(生长率:-77.10和-92.13,分别在10μM浓度)对来自肾癌的UO-31细胞系,并在五种不同浓度的10倍稀释液(10进一步选择用于分析-4至 10 -8 M)。化合物5a和5c 均表现出对白血病癌细胞系 HL-60 和 RPMI-8226、结肠癌细胞系 KM-12 的有希望的抗增殖活性(GI 50:1.36 至 0.27 μM);乳腺癌细胞系 BT-549。此外,发现化合物5a和5c对 HL-60、RPMI-8226 和 KM-12 细胞系均无细胞毒性(LC 50 > 100)。值得注意的是,GI化合物5a和5c 的50 个值被确定为比舒尼替尼更有希望对抗大多数癌细胞系。化合物5a和5c 的计算机模拟研究举例说明了所需的 ADME 特性,用于药物相似性以及与 VEGFR-2 的更紧密相互作用。因此,经过进一步的临床研究,化合物5a和5c将是很好的细胞毒剂。
更新日期:2021-10-19
中文翻译:

3-三氟甲基吡唑系链查尔酮-吡咯和吡唑啉-吡咯衍生物的合成、表征和生物学研究
本研究说明了新系列 3-三氟甲基吡唑系链查尔酮-吡咯和吡唑啉-吡咯衍生物的设计和合成。在美国国家癌症研究所进一步筛选所有化合物对完整 NCI 60 癌细胞系的体外细胞抑制活性。化合物( 2E )-3-( 1H-吡咯-2-基)-1-{4-[3-(三氟甲基) -1H-吡唑-1-基]苯基}prop-2-en-1-一( 5a )和( 2E )-1-{3-甲基-4-[3-(三氟甲基) -1H-吡唑-1-基]苯基}-3-( 1H-吡咯-2-基) prop-2-en-1-one ( 5c)显示显著的抗增殖活性(生长率:-77.10和-92.13,分别在10μM浓度)对来自肾癌的UO-31细胞系,并在五种不同浓度的10倍稀释液(10进一步选择用于分析-4至 10 -8 M)。化合物5a和5c 均表现出对白血病癌细胞系 HL-60 和 RPMI-8226、结肠癌细胞系 KM-12 的有希望的抗增殖活性(GI 50:1.36 至 0.27 μM);乳腺癌细胞系 BT-549。此外,发现化合物5a和5c对 HL-60、RPMI-8226 和 KM-12 细胞系均无细胞毒性(LC 50 > 100)。值得注意的是,GI化合物5a和5c 的50 个值被确定为比舒尼替尼更有希望对抗大多数癌细胞系。化合物5a和5c 的计算机模拟研究举例说明了所需的 ADME 特性,用于药物相似性以及与 VEGFR-2 的更紧密相互作用。因此,经过进一步的临床研究,化合物5a和5c将是很好的细胞毒剂。


























 京公网安备 11010802027423号
京公网安备 11010802027423号