Chemical Geology ( IF 3.9 ) Pub Date : 2021-08-18 , DOI: 10.1016/j.chemgeo.2021.120488 Xiangchong Liu 1, 2, 3 , Changhao Xiao 1, 2, 3 , Yong Wang 1, 2, 3
|
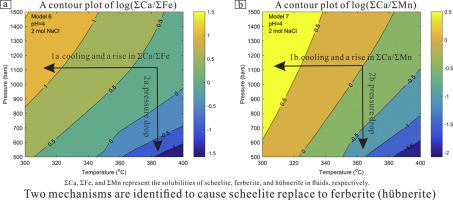
The occurrence of either scheelite (CaWO4) or wolframite ([Fe,Mn]WO4) in tungsten deposits is generally explained by the Ca content of the host rocks, but scheelite/wolframite ratios in granitoids-hosted tungsten deposits are highly variable, ranging from scheelite-dominated to wolframite-dominated. Wolframite is also commonly replaced by scheelite or vice versa- among many granitoids-hosted tungsten deposits. To fill this gap in understanding the stabilities of wolframite and scheelite under hydrothermal conditions, the relative solubilities of scheelite and wolframite in hydrothermal fluids were constrained using two modeled chemical systems: W-Ca-Mn-Cl-Na-O-H and W-Ca-Fe-Cl-Na-O-H. The two modeled chemical systems can well reproduce the available experimental data for scheelite, ferberite, and hübnerite solubilities under hydrothermal conditions. The modeling results indicate that scheelite is more soluble than hübnerite and ferberite under most W-mineralizing conditions (300–400 °C, 500–1500 bars, and 1–3 mol/kg NaCl). Scheelite can replace ferberite (hübnerite) over a wide range of temperatures and pressures; therefore, the replacement of ferberite (hübnerite) by scheelite does not necessarily represent late stages of mineralization. Two mechanisms can cause scheelite to replace ferberite (hübnerite). The first mechanism is cooling with an increase in the () molality ratio in fluids, and the second mechanism is a decrease in fluid pressure with constant (). Neither leaching Ca from Ca-rich wallrocks nor removing Fe (Mn) from hydrothermal fluids is required for the second mechanism. The second mechanism may account for vein-type or disseminated scheelite mineralization in host rocks whose Ca is low compared to carbonates.
中文翻译:

黑钨矿和白钨矿在热液中的相对溶解度:来自热力学模型的见解
白钨矿 (CaWO 4 ) 或黑钨矿 ([Fe,Mn]WO 4) 在钨矿床中通常由母岩的 Ca 含量来解释,但在花岗岩为主的钨矿床中白钨矿/黑钨矿的比率变化很大,范围从白钨矿为主到黑钨矿为主。在许多以花岗岩为主体的钨矿床中,黑钨矿通常也被白钨矿取代,反之亦然。为了填补在热液条件下了解黑钨矿和白钨矿的稳定性方面的这一空白,白钨矿和黑钨矿在热液中的相对溶解度受到两种模拟化学系统的约束:W-Ca-Mn-Cl-Na-OH 和 W-Ca- Fe-Cl-Na-OH。这两个模拟化学系统可以很好地再现白钨矿、铁镁矿和辉沸石在水热条件下溶解度的可用实验数据。建模结果表明,在大多数 W 矿化条件(300-400 °C、500-1500 bar 和 1-3 mol/kg NaCl)下,白钨矿比辉锰矿和铁镁矿更易溶解。白钨矿可以在很宽的温度和压力范围内替代铁碱矿(hübnerite);因此,白钨矿取代铁镁矿(hübnerite)并不一定代表矿化的后期阶段。两种机制可以导致白钨矿取代铁碱矿(hübnerite)。第一个机制是冷却 两种机制可以导致白钨矿取代铁碱矿(hübnerite)。第一个机制是冷却 两种机制可以导致白钨矿取代铁碱矿(hübnerite)。第一个机制是冷却 () 流体中的摩尔质量比,第二种机制是流体压力随着恒定的 ()。第二种机制既不需要从富含钙的围岩中浸出钙,也不需要从热液中去除铁 (Mn)。第二种机制可能解释了与碳酸盐相比 Ca 含量低的母岩中的脉状或浸染白钨矿化。


























 京公网安备 11010802027423号
京公网安备 11010802027423号