当前位置:
X-MOL 学术
›
Eur. J. Nerosci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Interplay between NMDA receptor dynamics and the synaptic proteasome
European Journal of Neroscience ( IF 3.4 ) Pub Date : 2021-08-17 , DOI: 10.1111/ejn.15427 Joana S. Ferreira 1 , Blanka Kellermayer 1, 2 , Ana Luísa Carvalho 2 , Laurent Groc 1
European Journal of Neroscience ( IF 3.4 ) Pub Date : 2021-08-17 , DOI: 10.1111/ejn.15427 Joana S. Ferreira 1 , Blanka Kellermayer 1, 2 , Ana Luísa Carvalho 2 , Laurent Groc 1
Affiliation
|
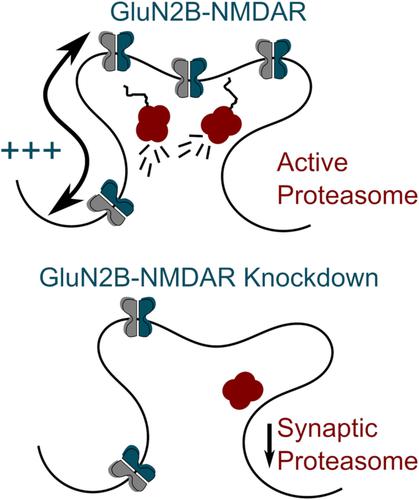
Proteasome activity at the excitatory synapse plays an important role in neuronal communication. The proteasome translocation to synapses is mediated by neuronal activity, in particular the activation of N-methyl-d-aspartate receptors (NMDARs). These receptors are composed of different subunits with distinct trafficking properties that provide various signalling and plasticity features to the synapse. Yet whether the interplay between the proteasome and NMDAR relies on specific subunit properties remain unclear. Using a combination of single molecule and immunocytochemistry imaging approaches in rat hippocampal neurons, we unveil a specific interplay between GluN2B-containing NMDARs (GluN2B-NMDARs) and the synaptic proteasome. Sustained proteasome activation specifically increases GluN2B-NMDAR (not GluN2A-NMDAR) lateral diffusion. In addition, when GluN2B-NMDAR expression is downregulated, the proteasome localization decreases at glutamatergic synapses. Collectively, our data fuel a model in which the cellular dynamics and location of GluN2B-NMDARs and proteasome are intermingled, shedding new lights on the NMDAR-dependent regulation of synaptic adaptation.
中文翻译:

NMDA 受体动力学与突触蛋白酶体之间的相互作用
兴奋性突触的蛋白酶体活性在神经元通讯中起着重要作用。蛋白酶体向突触的易位是由神经元活动介导的,特别是N-甲基-d的激活-天冬氨酸受体(NMDARs)。这些受体由具有不同运输特性的不同亚基组成,为突触提供各种信号传导和可塑性特征。然而,蛋白酶体和 NMDAR 之间的相互作用是否依赖于特定的亚基特性仍不清楚。在大鼠海马神经元中使用单分子和免疫细胞化学成像方法的组合,我们揭示了含有 GluN2B 的 NMDARs (GluN2B-NMDARs) 和突触蛋白酶体之间的特定相互作用。持续的蛋白酶体激活特别增加了 GluN2B-NMDAR(不是 GluN2A-NMDAR)横向扩散。此外,当 GluN2B-NMDAR 表达下调时,蛋白酶体定位在谷氨酸能突触处降低。总的来说,
更新日期:2021-09-21
中文翻译:

NMDA 受体动力学与突触蛋白酶体之间的相互作用
兴奋性突触的蛋白酶体活性在神经元通讯中起着重要作用。蛋白酶体向突触的易位是由神经元活动介导的,特别是N-甲基-d的激活-天冬氨酸受体(NMDARs)。这些受体由具有不同运输特性的不同亚基组成,为突触提供各种信号传导和可塑性特征。然而,蛋白酶体和 NMDAR 之间的相互作用是否依赖于特定的亚基特性仍不清楚。在大鼠海马神经元中使用单分子和免疫细胞化学成像方法的组合,我们揭示了含有 GluN2B 的 NMDARs (GluN2B-NMDARs) 和突触蛋白酶体之间的特定相互作用。持续的蛋白酶体激活特别增加了 GluN2B-NMDAR(不是 GluN2A-NMDAR)横向扩散。此外,当 GluN2B-NMDAR 表达下调时,蛋白酶体定位在谷氨酸能突触处降低。总的来说,


























 京公网安备 11010802027423号
京公网安备 11010802027423号