Cell Calcium ( IF 4 ) Pub Date : 2021-08-17 , DOI: 10.1016/j.ceca.2021.102454 Sharon Negri 1 , Pawan Faris 1 , Claudia Maniezzi 1 , Giorgia Pellavio 2 , Paolo Spaiardi 1 , Laura Botta 1 , Umberto Laforenza 2 , Gerardo Biella 1 , Dr Francesco Moccia 1
|
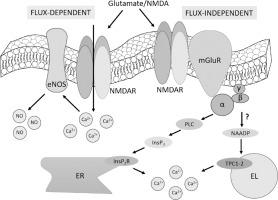
The excitatory neurotransmitter glutamate gates post-synaptic N-methyl-d-aspartate (NMDA) receptors (NMDARs) to mediate extracellular Ca2+ entry and stimulate neuronal nitric oxide (NO) synthase to release NO and trigger neurovascular coupling (NVC). Neuronal and glial NMDARs may also operate in a flux-independent manner, although it is unclear whether their non-ionotropic mode of action is involved in NVC. Recently, endothelial NMDARs were found to trigger Ca2+-dependent NO production and induce NVC, but the underlying mode of signaling remains elusive. Herein, we report that GluN1 protein, as well as GluN2C and GluN3B transcripts and proteins, were expressed and that NMDA did not elicit inward currents, but induced a dose-dependent increase in intracellular Ca2+ concentration ([Ca2+]i) in the human brain microvascular endothelial cell line, hCMEC/D3. A multidisciplinary approach, including live cell imaging, whole-cell patch-clamp recordings, pharmacological manipulation and gene targeting, revealed that NMDARs increase the [Ca2+]i in a flux-independent manner in hCMEC/D3 cells. The Ca2+ response to NMDA was triggered by endogenous Ca2+ release from the endoplasmic reticulum and the lysosomal Ca2+ stores and sustained by store-operated Ca2+ entry. Unexpectedly, pharmacological and genetic blockade of mGluR1 and mGluR5 dramatically impaired NMDARs-mediated Ca2+ signals. These findings indicate that NMDARs may increase the endothelial [Ca2+]i in a flux-independent manner via group 1 mGluRs. However, imaging of DAF-FM fluorescence revealed that NMDARs may also induce Ca2+-dependent NO release by signaling in a flux-dependent manner. These findings, therefore, shed novel light on the mechanisms whereby brain microvascular endothelium decodes glutamatergic signaling and regulates NVC.
中文翻译:

NMDA 受体通过代谢型谷氨酸受体和通量依赖性一氧化氮释放在人脑微血管内皮细胞中引发不依赖通量的细胞内 Ca2+ 信号
兴奋性神经递质谷氨酸门控突触后 N-甲基-d-天冬氨酸 (NMDA) 受体 (NMDAR) 介导细胞外 Ca 2+进入并刺激神经元一氧化氮 (NO) 合酶释放 NO 并触发神经血管耦合 (NVC)。神经元和神经胶质 NMDAR 也可能以与通量无关的方式运行,尽管尚不清楚它们的非离子作用模式是否参与 NVC。最近,发现内皮 NMDARs 触发 Ca 2+依赖 NO 产生并诱导 NVC,但信号传导的潜在模式仍然难以捉摸。在此,我们报告了 GluN1 蛋白以及 GluN2C 和 GluN3B 转录物和蛋白质的表达,并且 NMDA 不会引起内向电流,但会诱导细胞内 Ca 2+浓度的剂量依赖性增加([Ca 2+ ] i)在人脑微血管内皮细胞系 hCMEC/D3 中。包括活细胞成像、全细胞膜片钳记录、药理学操作和基因靶向在内的多学科方法表明,NMDARs 在 hCMEC/D3 细胞中以与通量无关的方式增加 [Ca 2+ ] i。对 NMDA的 Ca 2+反应是由内源性 Ca 触发的2+从内质网释放和溶酶体 Ca 2+储存并由储存操作的 Ca 2+进入维持。出乎意料的是,mGluR1 和 mGluR5 的药理学和遗传阻断显着损害了 NMDARs 介导的 Ca 2+信号。这些发现表明,NMDARs 可以通过第 1 组 mGluRs 以不依赖于通量的方式增加内皮细胞 [Ca 2+ ] i 。然而,DAF-FM 荧光成像显示,NMDAR 还可以通过以通量依赖性方式发出信号来诱导 Ca 2+依赖性 NO 释放。因此,这些发现揭示了脑微血管内皮解码谷氨酸能信号和调节 NVC 的机制。


























 京公网安备 11010802027423号
京公网安备 11010802027423号