Journal of Environmental Chemical Engineering ( IF 7.7 ) Pub Date : 2021-08-17 , DOI: 10.1016/j.jece.2021.106220 Ahmed S.A.A. Abu Sharib 1 , Adrián Bonilla-Petriciolet 2 , Ali Q. Selim 3 , Essam A. Mohamed 3 , Moaaz K. Seliem 3
|
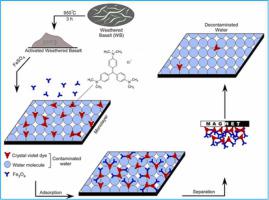
Weathered basalt activated at 950 ⁰C for 3h was used, for the first time, as a source of Fe(III) to prepare magnetic Fe3O4 nanoparticles (MNPs). The synthesized Fe3O4 MNPs were characterized by XRD, FTIR, FESEM, and TEM and utilized as a cost effective adsorbent for the removal of crystal violet (CV) dye at pH 7.0 and 25, 40, and 50 °C. The experimental data were fitted to the Langmuir model with maximum adsorption capacities that ranged from 269.7 to 282.5 mg/g, and increased with the solution temperature. Three theoretical advanced models from statistical physics theory were used to interpret the corresponding adsorption mechanism. It was concluded that the single layer model, with one active site, was the best adsorption model for this system. The number of adsorbed CV molecules per the adsorption site (n) was 1.2 – 1.6 suggesting the existence of multi–interactions mechanism. The density of Fe3O4 active sites (DM) was the main steric factor governing the removal process, and this parameter improved from 134.7 to 218.33 mg/g consistent with the increase of the adsorption temperature. The adsorption energy was below 40 kJ/mol and, hence, the removal of CV molecules was directed mainly by physical forces. The low cost, regeneration performance, and chemical stability of these Fe3O4 MNPs suggest their application as a very promising approach for wastewater treatment. The results present a new approach in the fabrication of magnetic nanoparticles using Fe-rich natural materials, as alternative sources for iron compounds, to be employed in water and wastewater remediation.
effective adsorbent for the removal of crystal violet (CV) dye at pH 7.0 and 25, 40, and 50 °C. The experimental data were fitted to the Langmuir model with maximum adsorption capacities that ranged from 269.7 to 282.5 mg/g, and increased with the solution temperature. Three theoretical advanced models from statistical physics theory were used to interpret the corresponding adsorption mechanism. It was concluded that the single layer model, with one active site, was the best adsorption model for this system. The number of adsorbed CV molecules per the adsorption site (n) was 1.2 – 1.6 suggesting the existence of multi–interactions mechanism. The density of Fe3O4 active sites (DM) was the main steric factor governing the removal process, and this parameter improved from 134.7 to 218.33 mg/g consistent with the increase of the adsorption temperature. The adsorption energy was below 40 kJ/mol and, hence, the removal of CV molecules was directed mainly by physical forces. The low cost, regeneration performance, and chemical stability of these Fe3O4 MNPs suggest their application as a very promising approach for wastewater treatment. The results present a new approach in the fabrication of magnetic nanoparticles using Fe-rich natural materials, as alternative sources for iron compounds, to be employed in water and wastewater remediation.
中文翻译:

利用改性风化玄武岩作为制备 Fe3O4 纳米颗粒的新方法:结晶紫吸附的实验和理论研究
在 950 ⁰C 下活化 3 小时的风化玄武岩首次用作 Fe(III) 的来源,以制备磁性 Fe 3 O 4纳米粒子 (MNP)。合成的 Fe 3 O 4 MNPs 通过 XRD、FTIR、FESEM 和 TEM 进行表征,并用作成本 在 pH 7.0 和 25、40 和 50 °C 下去除结晶紫 (CV) 染料的有效吸附剂。实验数据符合 Langmuir 模型,最大吸附容量范围为 269.7 至 282.5 毫克/克,并随着溶液温度的增加而增加。来自统计物理理论的三个理论高级模型被用来解释相应的吸附机制。结论是具有一个活性位点的单层模型是该系统的最佳吸附模型。每吸附位点(吸附CV分子数Ñ)为1.2 - 1.6提示的多相互作用机制的存在。Fe 3 O 4活性位点的密度(D M) 是控制去除过程的主要空间因素,该参数从 134.7 提高到 218.33 mg/g,与吸附温度的升高一致。吸附能低于 40 kJ/mol,因此,CV 分子的去除主要由物理力引导。这些 Fe 3 O 4 MNP 的低成本、再生性能和化学稳定性表明它们是一种非常有前途的废水处理方法。结果提出了一种使用富铁天然材料制造磁性纳米粒子的新方法,作为铁化合物的替代来源,用于水和废水修复。
在 pH 7.0 和 25、40 和 50 °C 下去除结晶紫 (CV) 染料的有效吸附剂。实验数据符合 Langmuir 模型,最大吸附容量范围为 269.7 至 282.5 毫克/克,并随着溶液温度的增加而增加。来自统计物理理论的三个理论高级模型被用来解释相应的吸附机制。结论是具有一个活性位点的单层模型是该系统的最佳吸附模型。每吸附位点(吸附CV分子数Ñ)为1.2 - 1.6提示的多相互作用机制的存在。Fe 3 O 4活性位点的密度(D M) 是控制去除过程的主要空间因素,该参数从 134.7 提高到 218.33 mg/g,与吸附温度的升高一致。吸附能低于 40 kJ/mol,因此,CV 分子的去除主要由物理力引导。这些 Fe 3 O 4 MNP 的低成本、再生性能和化学稳定性表明它们是一种非常有前途的废水处理方法。结果提出了一种使用富铁天然材料制造磁性纳米粒子的新方法,作为铁化合物的替代来源,用于水和废水修复。


























 京公网安备 11010802027423号
京公网安备 11010802027423号