Current Analytical Chemistry ( IF 1.8 ) Pub Date : 2021-09-30 , DOI: 10.2174/1573411017999210112175743 J Fan 1 , P P Cheney 1 , S Bloch 1 , B Xu 1 , K Liang 1 , C A Odonkor 1 , W B Edwards 1 , S Basak 2 , R Mintz 1, 2, 3, 4, 5 , P Biswas 2 , S Achilefu 1, 3, 4, 5
|
Background: Gold nanoparticles (AuNPs) are commonly used in nanomedicine because of their unique spectral properties, chemical and biological stability, and ability to quench the fluorescence of organic dyes attached to their surfaces. However, the utility of spherical AuNPs for activatable fluorescence sensing of molecular processes have been confined to resonance-matched fluorophores in the 500 nm to 600 nm spectral range to maximize dye fluorescence quenching efficiency. Expanding the repertoire of fluorophore systems into the NIR fluorescence regimen with emission >800 nm will facilitate the analysis of multiple biological events with high detection sensitivity.
Objective: The primary goal of this study is to determine if spherical AuNP-induced radiative rate suppression of non-resonant near-infrared (NIR) fluorescent probes can serve as a versatile nanoconstruct for highly sensitive detection and imaging of activated caspase-3 in aqueous media and cancer cells. This required the development of activatable NIR fluorescence sensors of caspase-3 designed to overcome the nonspecific degradation and release of the surface coatings in aqueous media.
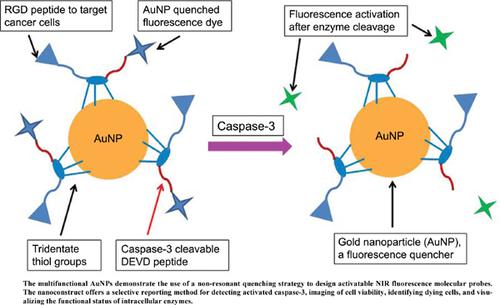
Methods: We harnessed the fluorescence-quenching properties and multivalency of spherical AuNPs to develop AuNP-templated activatable NIR fluorescent probes to detect activated caspase-3, an intracellular reporter of early cell death. Freshly AuNPs were coated with a multifunctional NIR fluorescent dye-labeled peptide (LS422) consisting of an RGD peptide sequence that targets αvβ3-integrin protein (αvβ3) on the surface of cancer cells to mediate the uptake and internalization of the sensors in tumor cells; a DEVD peptide sequence for reporting the induction of cell death through caspase-3 mediated NIR fluorescence enhancement; and a multidentate hexacysteine sequence for enhancing self-assembly and stabilizing the multifunctional construct on AuNPs. The integrin-binding affinity of LS422 and caspase-3 kinetics were determined by competitive radioligand binding and fluorogenic peptide assays, respectively. Detection of intracellular caspase-3, cell viability, and the internalization of LS422 in cancer cells was determined by confocal NIR fluorescence spectroscopy and microscopy.
Results: Narrow size AuNPs (13 nm) were prepared and characterized by transmission electron microscopy and dynamic light scattering. When assembled on the AuNPs, the binding constant of LS422 for αvβ3 improved 11- fold from 13.2 nM to 1.2 nM. Whereas the catalytic turnover of caspase-3 by LS422-AuNPs was similar to the reference fluorogenic peptide, the binding affinity for the enzyme increased by a factor of 2. Unlike the αvβ3 positive, but caspase-3 negative breast cancer MCF-7 cells, treatment of the αvβ3 and caspase-3 positive lung cancer A549 cells with Paclitaxel showed significant fluorescence enhancement within 30 minutes, which correlated with caspase-3 specific activation of LS422-AuNPs fluorescence. The incorporation of a 3.5 mW NIR laser source into our spectrofluorometer increased the detection sensitivity by an order of magnitude (limit of detection ~0.1 nM of cypate) and significantly decreased the signal noise relative to a xenon lamp. This gain in sensitivity enabled the detection of substrate hydrolysis at a broad range of inhibitor concentrations without photobleaching the cypate dye.
Conclusion: The multifunctional AuNPs demonstrate the use of a non-resonant quenching strategy to design activatable NIR fluorescence molecular probes. The nanoconstruct offers a selective reporting method for detecting activated caspase-3, imaging of cell viability, identifying dying cells, and visualizing the functional status of intracellular enzymes. Performing these tasks with NIR fluorescent probes creates an opportunity to translate the in vitro and cellular analysis of enzymes into in vivo interrogation of their functional status using deep tissue penetrating NIR fluorescence analytical methods.
中文翻译:

用于活化 Caspase-3 的近红外荧光检测和成像的多功能硫稳定金纳米粒子
背景:金纳米粒子 (AuNPs) 因其独特的光谱特性、化学和生物稳定性以及淬灭附着在其表面的有机染料荧光的能力而常用于纳米医学。然而,球形金纳米粒子用于分子过程的可激活荧光传感的效用仅限于 500 nm 至 600 nm 光谱范围内的共振匹配荧光团,以最大限度地提高染料荧光猝灭效率。将荧光团系统的所有组成部分扩展到发射 > 800 nm 的 NIR 荧光方案中,将有助于以高检测灵敏度分析多种生物事件。
目的:本研究的主要目的是确定球形 AuNP 诱导的非共振近红外 (NIR) 荧光探针的辐射率抑制是否可以作为一种多功能纳米结构,用于高灵敏度检测和成像水溶液中的活化 caspase-3培养基和癌细胞。这需要开发可激活的 Caspase-3 近红外荧光传感器,旨在克服水介质中表面涂层的非特异性降解和释放。
方法:我们利用球形金纳米粒子的荧光猝灭特性和多价性来开发以金纳米粒子为模板的可激活 NIR 荧光探针,以检测活化的 caspase-3,一种早期细胞死亡的细胞内报告基因。新鲜的 AuNPs 涂有多功能 NIR 荧光染料标记肽 (LS422),该肽由靶向 α v β 3 -整合素蛋白 (α v β 3 ) 的 RGD 肽序列组成) 在癌细胞表面介导肿瘤细胞中传感器的摄取和内化;用于报告通过 caspase-3 介导的 NIR 荧光增强诱导细胞死亡的 DEVD 肽序列;以及用于增强自组装和稳定 AuNPs 上的多功能构建体的多齿六半胱氨酸序列。LS422 的整合素结合亲和力和 caspase-3 动力学分别通过竞争性放射性配体结合和荧光肽测定来确定。细胞内 caspase-3、细胞活力和 LS422 在癌细胞中的内化的检测通过共聚焦 NIR 荧光光谱和显微镜检查来确定。
结果:制备了窄尺寸的金纳米粒子(13 nm),并通过透射电子显微镜和动态光散射对其进行了表征。当在 AuNPs 上组装时,LS422 对 αvβ3 的结合常数提高了 11 倍,从 13.2 nM 提高到 1.2 nM。虽然 LS422-AuNPs 对 caspase-3 的催化转换与参考荧光肽相似,但对酶的结合亲和力增加了 2 倍。与 αvβ3 阳性但 caspase-3 阴性乳腺癌 MCF-7 细胞不同,用紫杉醇处理 αvβ3 和 caspase-3 阳性肺癌 A549 细胞在 30 分钟内显示出显着的荧光增强,这与 caspase-3 特异性激活 LS422-AuNPs 荧光有关。3. 合并。5 mW NIR 激光源进入我们的分光荧光计将检测灵敏度提高了一个数量级(检测限约 0.1 nM cypate),并显着降低了相对于氙灯的信号噪声。这种灵敏度的提高能够在广泛的抑制剂浓度范围内检测底物水解,而不会光漂白 cypate 染料。
结论:多功能 AuNPs 证明了使用非共振猝灭策略来设计可激活的 NIR 荧光分子探针。该纳米结构提供了一种选择性报告方法,用于检测活化的 caspase-3、细胞活力成像、识别垂死细胞和可视化细胞内酶的功能状态。使用 NIR 荧光探针执行这些任务创造了一个机会,可以使用深层组织穿透 NIR 荧光分析方法将酶的体外和细胞分析转化为对其功能状态的体内询问。


























 京公网安备 11010802027423号
京公网安备 11010802027423号