Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Mechanical Stimulation after Centrifuge-Free Nano-Electroporative Transfection Is Efficient and Maintains Long-Term T Cell Functionalities
Small ( IF 13.3 ) Pub Date : 2021-08-15 , DOI: 10.1002/smll.202103198 Andy Tay 1, 2 , Nicholas Melosh 3
Small ( IF 13.3 ) Pub Date : 2021-08-15 , DOI: 10.1002/smll.202103198 Andy Tay 1, 2 , Nicholas Melosh 3
Affiliation
|
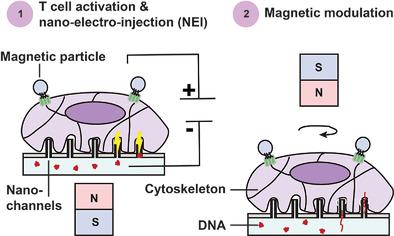
Transfection is an essential step in genetic engineering and cell therapies. While a number of non-viral micro- and nano-technologies have been developed to deliver DNA plasmids into the cell cytoplasm, one of the most challenging and least efficient steps is DNA transport to and expression in the nucleus. Here, the magnetic nano-electro-injection (MagNEI) platform is described which makes use of oscillatory mechanical stimulation after cytoplasmic delivery with high aspect-ratio nano-structures to achieve stable (>2 weeks) net transfection efficiency (efficiency × viability) of 50% in primary human T cells. This is, to the best of the authors’ knowledge, the highest net efficiency reported for primary T cells using a centrifuge-free, non-viral transfection method, in the absence of cell selection, and with a clinically relevant cargo size (>12 kbp). Wireless mechanical stimulation downregulates the expression of microtubule motor protein gene, KIF2A, which increases local DNA concentration near the nuclei, resulting in enhanced DNA transfection. Magnetic forces also accelerate membrane repair by promoting actin cytoskeletal remodeling which preserves key biological attributes including cell proliferation and gene expressions. These results demonstrate MagNEI as a powerful non-viral transfection technique for progress toward fully closed, end-to-end T cell manufacturing with less human labor, lower production cost, and shorter delay.
中文翻译:

免离心纳米电穿孔转染后的机械刺激有效并保持长期 T 细胞功能
转染是基因工程和细胞治疗的重要步骤。虽然已经开发了许多非病毒微米和纳米技术来将 DNA 质粒递送到细胞质中,但最具挑战性和效率最低的步骤之一是将 DNA 转运到细胞核并在细胞核中表达。在此,描述了磁性纳米电注射(MagNEI)平台,该平台利用高纵横比纳米结构的细胞质递送后的振荡机械刺激来实现稳定(>2周)的净转染效率(效率×活力) 50% 存在于原代人类 T 细胞中。据作者所知,这是使用无离心、非病毒转染方法、在没有细胞选择的情况下、具有临床相关货物大小(>12)的原代 T 细胞报告的最高净效率。 kbp)。无线机械刺激下调微管运动蛋白基因KIF2A的表达,从而增加细胞核附近的局部 DNA 浓度,从而增强 DNA 转染。磁力还通过促进肌动蛋白细胞骨架重塑来加速膜修复,从而保留细胞增殖和基因表达等关键生物属性。这些结果表明 MagNEI 是一种强大的非病毒转染技术,可实现全封闭、端到端 T 细胞制造,并减少人力、降低生产成本和缩短延迟。
更新日期:2021-09-23
中文翻译:

免离心纳米电穿孔转染后的机械刺激有效并保持长期 T 细胞功能
转染是基因工程和细胞治疗的重要步骤。虽然已经开发了许多非病毒微米和纳米技术来将 DNA 质粒递送到细胞质中,但最具挑战性和效率最低的步骤之一是将 DNA 转运到细胞核并在细胞核中表达。在此,描述了磁性纳米电注射(MagNEI)平台,该平台利用高纵横比纳米结构的细胞质递送后的振荡机械刺激来实现稳定(>2周)的净转染效率(效率×活力) 50% 存在于原代人类 T 细胞中。据作者所知,这是使用无离心、非病毒转染方法、在没有细胞选择的情况下、具有临床相关货物大小(>12)的原代 T 细胞报告的最高净效率。 kbp)。无线机械刺激下调微管运动蛋白基因KIF2A的表达,从而增加细胞核附近的局部 DNA 浓度,从而增强 DNA 转染。磁力还通过促进肌动蛋白细胞骨架重塑来加速膜修复,从而保留细胞增殖和基因表达等关键生物属性。这些结果表明 MagNEI 是一种强大的非病毒转染技术,可实现全封闭、端到端 T 细胞制造,并减少人力、降低生产成本和缩短延迟。



























 京公网安备 11010802027423号
京公网安备 11010802027423号