Chemical Engineering Journal ( IF 15.1 ) Pub Date : 2021-08-14 , DOI: 10.1016/j.cej.2021.131770 Tingsheng Zhou 1 , Lei Li 1 , Jinhua Li 1 , Jiachen Wang 1 , Jing Bai 1, 2 , Ligang Xia 3, 4 , Qunjie Xu 3, 4 , Baoxue Zhou 1, 2, 4
|
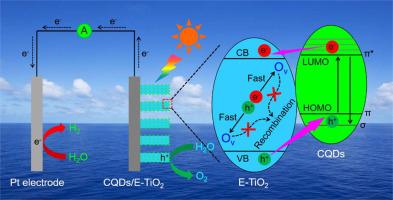
Severe bulk charge recombination, sluggish oxygen evolution rection (OER) kinetics and poor visible light harvesting are still the technical bottlenecks of famous TiO2 photoanode for photoelectrochemical (PEC) water splitting. Here, a novel CQDs/E-TiO2 photoanode was designed based on the accurately electrochemical reduction of TiO2 (E-TiO2, Ti4+ + e- → Ti3+) and further modification of oxygen vacancy (OV)-rich carbon quantum dots (CQDs) for synergistically improving PEC performance. The electrochemical reduction creates modreate OV in TiO2, which increase the majority carrier density and provide photoinduced charge traps for sharply increasing the bulk charge separation efficiency (ηbulk). The CQDs modification dramatically improves the surface charge transfer efficiency (ηsurface) by serving as oxygen evolution catalysts (OECs), because the abundant OV in CQDs greatly promote the OER kinetics. Additionally, the visible light harvesting of TiO2 is significantly improved after CQDs modification. Near-complete bulk charge separation (ηbulk = 94.7%) is achieved for E-TiO2 at 1.23 V vs. RHE (VRHE), which is 4.0 times higher than that of TiO2. The CQDs/E-TiO2 shows the ηsurface of 56.0% at 0.40 VRHE, which is 7.2 times higher than E-TiO2. Therefore, the CQDs/E-TiO2 exhibits remarkable photocurrent densities of 1.50 mA cm-2 at 0.60 VRHE and 2.55 mA cm-2 at 1.23 VRHE, which are 27.0 and 10.0 times higher than TiO2, 3.5 and 1.5 times higher than E-TiO2, respectively.
中文翻译:

电化学还原的 TiO2 光阳极与富氧空位碳量子点相结合,协同提高光电化学性能
严重的体电荷复合、缓慢的析氧反应(OER)动力学和较差的可见光捕获仍然是著名的用于光电化学(PEC)水分解的TiO 2 光阳极的技术瓶颈。在此,基于 TiO 2 (E-TiO 2 , Ti 4+ + e - → Ti 3+ )的精确电化学还原和氧空位 ( OV )- 的进一步改性,设计了一种新型 CQDs/E-TiO 2 光阳极富碳量子点 (CQD) 可协同提高 PEC 性能。电化学还原在 TiO 2 中产生适度的 O V,这增加了多数载流子密度和用于急剧提高堆积的电荷分离效率(光诱导提供电荷陷阱η散装)。该变形例CQDs显着地提高表面电荷转移效率(η表面由作为析氧催化剂(嗅鞘细胞)),因为丰富Ó V在CQDs极大地促进了OER动力学。此外,在 CQDs 改性后,TiO 2的可见光捕获得到显着改善。接近完全散装电荷分离(η散装= 94.7%)为E-的TiO实现2 1.23 V,相对于RHE(V RHE),这比的TiO更高4.0倍2. CQDs/E-TiO 2在0.40 V RHE 下显示56.0%的η表面,比E-TiO 2高7.2倍。因此,CQDs/E-TiO 2表现出显着的光电流密度,在 0.60 V RHE 下为 1.50 mA cm -2,在 1.23 V RHE 下为2.55 mA cm -2,分别是 TiO 2 的27.0 和 10.0 倍,分别高出3.5 和 1.5 倍分别比 E-TiO 2。


























 京公网安备 11010802027423号
京公网安备 11010802027423号