Advanced Drug Delivery Reviews ( IF 16.1 ) Pub Date : 2021-08-14 , DOI: 10.1016/j.addr.2021.113929 Michael S Roberts 1 , Hanumanth S Cheruvu 2 , Sean E Mangion 3 , Azadeh Alinaghi 4 , Heather A E Benson 5 , Yousuf Mohammed 2 , Amy Holmes 4 , John van der Hoek 6 , Michael Pastore 7 , Jeffrey E Grice 2
|
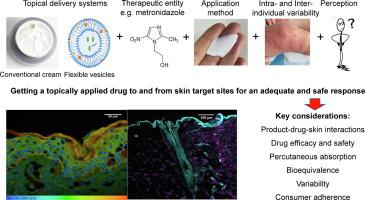
Topical products, widely used to manage skin conditions, have evolved from simple potions to sophisticated delivery systems. Their development has been facilitated by advances in percutaneous absorption and product design based on an increasingly mechanistic understanding of drug-product-skin interactions, associated experiments, and a quality-by-design framework. Topical drug delivery involves drug transport from a product on the skin to a local target site and then clearance by diffusion, metabolism, and the dermal circulation to the rest of the body and deeper tissues. Insights have been provided by Quantitative Structure Permeability Relationships (QSPR), molecular dynamics simulations, and dermal Physiologically Based PharmacoKinetics (PBPK). Currently, generic product equivalents of reference-listed products dominate the topical delivery market. There is an increasing regulatory interest in understanding topical product delivery behavior under ‘in use’ conditions and predicting in vivo response for population variations in skin barrier function and response using in silico and in vitro findings.
中文翻译:

局部给药:历史、经皮吸收和产品开发
广泛用于管理皮肤状况的外用产品已经从简单的药水演变为复杂的递送系统。基于对药物-产品-皮肤相互作用、相关实验和质量设计框架的日益机械化理解,经皮吸收和产品设计的进步促进了它们的发展。局部药物递送涉及药物从皮肤上的产品运输到局部目标部位,然后通过扩散、代谢和真皮循环清除到身体其他部位和更深的组织。定量结构渗透性关系 (QSPR)、分子动力学模拟和基于皮肤生理学的药代动力学 (PBPK) 提供了见解。目前,参考上市产品的仿制药等价物主导着局部给药市场。越来越多的监管机构对了解在“使用中”条件和预测皮肤屏障功能人群变化的体内反应和使用计算机和体外研究结果的反应。

























 京公网安备 11010802027423号
京公网安备 11010802027423号