当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Efficient Delivery of Chlorin e6 by Polyglycerol-Coated Iron Oxide Nanoparticles with Conjugated Doxorubicin for Enhanced Photodynamic Therapy of Melanoma
Molecular Pharmaceutics ( IF 4.9 ) Pub Date : 2021-08-13 , DOI: 10.1021/acs.molpharmaceut.1c00510 Tong-Fei Li 1, 2, 3, 4 , Hua-Zhen Xu 3, 4 , Yong-Hong Xu 5 , Ting-Ting Yu 1, 2 , Jun-Ming Tang 1, 2 , Ke Li 3, 4 , Chao Wang 3, 4 , Xing-Chun Peng 1, 2 , Qi-Rui Li 1, 2 , Xue-Yu Sang 1, 2 , Mei-Yan Zheng 3, 4 , Yan Liu 3, 4 , Li Zhao 6 , Xiao Chen 3, 4
Molecular Pharmaceutics ( IF 4.9 ) Pub Date : 2021-08-13 , DOI: 10.1021/acs.molpharmaceut.1c00510 Tong-Fei Li 1, 2, 3, 4 , Hua-Zhen Xu 3, 4 , Yong-Hong Xu 5 , Ting-Ting Yu 1, 2 , Jun-Ming Tang 1, 2 , Ke Li 3, 4 , Chao Wang 3, 4 , Xing-Chun Peng 1, 2 , Qi-Rui Li 1, 2 , Xue-Yu Sang 1, 2 , Mei-Yan Zheng 3, 4 , Yan Liu 3, 4 , Li Zhao 6 , Xiao Chen 3, 4
Affiliation
|
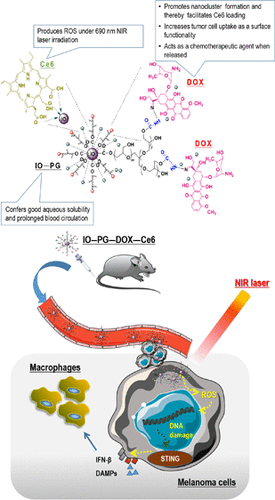
Chlorin e6 (Ce6) is a promising photosensitizer for tumor photodynamic therapy (PDT). However, the efficacy of Ce6 PDT is limited by Ce6’s poor water solubility, rapid blood clearance, and inadequate accumulation in the tumor tissue. This problem is tackled in this work, wherein functionalized superparamagnetic iron oxide nanoparticles (IO-NPs) were used as carriers to deliver Ce6 to melanoma. The IO-NPs were coated with polyglycerol (PG) to afford good aqueous solubility. The chemotherapeutic agent doxorubicin (DOX) was attached to the PG coating via the hydrazone bond to afford affinity to the cell membrane and thereby promote the cell uptake. The hydrophobic nature of DOX also induced the aggregation of IO-NPs to form nanoclusters. Ce6 was then loaded onto the IO nanoclusters through physical adsorption and coordination with surface iron atoms, yielding the final composites IO–PG–DOX–Ce6. In vitro experiments showed that IO–PG–DOX–Ce6 markedly increased Ce6 uptake in mouse melanoma cells, leading to much-enhanced photocytotoxicity characterized by intensified reactive oxygen species production, loss of viability, DNA damage, and stimulation of tumor cell immunogenicity. In vivo experiments corroborated the in vitro findings and demonstrated prolonged blood clearance of IO–PG–DOX–Ce6. Importantly, IO–PG–DOX–Ce6 markedly increased the Ce6 distribution and retention in mouse subcutaneous melanoma grafts and significantly improved the efficacy of Ce6-mediated PDT. No apparent vital organ damage was observed at the same time. In conclusion, the IO–PG–DOX NPs provide a simple and safe delivery platform for efficient tumor enrichment of Ce6, thereby enhancing antimelanoma PDT.
中文翻译:

聚甘油包覆的氧化铁纳米颗粒与共轭多柔比星有效递送氯离子 e6 以增强黑色素瘤的光动力治疗
Chlorin e6 (Ce6) 是一种用于肿瘤光动力疗法 (PDT) 的有前途的光敏剂。然而,Ce6 PDT 的疗效受到 Ce6 水溶性差、血液清除快、在肿瘤组织中积累不足的限制。这个问题在这项工作中得到了解决,其中功能化的超顺磁性氧化铁纳米颗粒 (IO-NPs) 被用作载体,将 Ce6 递送至黑色素瘤。IO-NPs 涂有聚甘油 (PG) 以提供良好的水溶性。化疗剂多柔比星 (DOX) 通过腙键附着在 PG 涂层上,以提供对细胞膜的亲和力,从而促进细胞摄取。DOX 的疏水性也诱导 IO-NP 聚集形成纳米团簇。然后通过物理吸附和与表面铁原子的配位将 Ce6 加载到 IO 纳米团簇上,产生最终的复合材料 IO-PG-DOX-Ce6。体外实验表明,IO-PG-DOX-Ce6 显着增加了小鼠黑色素瘤细胞对 Ce6 的摄取,导致光细胞毒性显着增强,其特征是活性氧产生增强、活力丧失、DNA 损伤和刺激肿瘤细胞免疫原性。体内实验证实了体外研究结果并证明了 IO-PG-DOX-Ce6 的血液清除时间延长。重要的是,IO-PG-DOX-Ce6 显着增加了 Ce6 在小鼠皮下黑色素瘤移植物中的分布和保留,并显着提高了 Ce6 介导的 PDT 的功效。同时没有观察到明显的重要器官损伤。总之,IO-PG-DOX NPs为Ce6的有效肿瘤富集提供了一个简单且安全的递送平台,从而增强了抗黑色素瘤PDT。
更新日期:2021-09-06
中文翻译:

聚甘油包覆的氧化铁纳米颗粒与共轭多柔比星有效递送氯离子 e6 以增强黑色素瘤的光动力治疗
Chlorin e6 (Ce6) 是一种用于肿瘤光动力疗法 (PDT) 的有前途的光敏剂。然而,Ce6 PDT 的疗效受到 Ce6 水溶性差、血液清除快、在肿瘤组织中积累不足的限制。这个问题在这项工作中得到了解决,其中功能化的超顺磁性氧化铁纳米颗粒 (IO-NPs) 被用作载体,将 Ce6 递送至黑色素瘤。IO-NPs 涂有聚甘油 (PG) 以提供良好的水溶性。化疗剂多柔比星 (DOX) 通过腙键附着在 PG 涂层上,以提供对细胞膜的亲和力,从而促进细胞摄取。DOX 的疏水性也诱导 IO-NP 聚集形成纳米团簇。然后通过物理吸附和与表面铁原子的配位将 Ce6 加载到 IO 纳米团簇上,产生最终的复合材料 IO-PG-DOX-Ce6。体外实验表明,IO-PG-DOX-Ce6 显着增加了小鼠黑色素瘤细胞对 Ce6 的摄取,导致光细胞毒性显着增强,其特征是活性氧产生增强、活力丧失、DNA 损伤和刺激肿瘤细胞免疫原性。体内实验证实了体外研究结果并证明了 IO-PG-DOX-Ce6 的血液清除时间延长。重要的是,IO-PG-DOX-Ce6 显着增加了 Ce6 在小鼠皮下黑色素瘤移植物中的分布和保留,并显着提高了 Ce6 介导的 PDT 的功效。同时没有观察到明显的重要器官损伤。总之,IO-PG-DOX NPs为Ce6的有效肿瘤富集提供了一个简单且安全的递送平台,从而增强了抗黑色素瘤PDT。


























 京公网安备 11010802027423号
京公网安备 11010802027423号