当前位置:
X-MOL 学术
›
J. Cell. Biochem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Exosomal tumor necrosis factor-α from hepatocellular cancer cells (Huh-7) promote osteoclast differentiation
Journal of Cellular Biochemistry ( IF 4 ) Pub Date : 2021-08-12 , DOI: 10.1002/jcb.30127 Ching-Hao Li, Kalaiselvi Palanisamy, Xin Li, Shao-Hua Yu, I-Kuan Wang, Chi-Yuan Li, Kuo-Ting Sun
Journal of Cellular Biochemistry ( IF 4 ) Pub Date : 2021-08-12 , DOI: 10.1002/jcb.30127 Ching-Hao Li, Kalaiselvi Palanisamy, Xin Li, Shao-Hua Yu, I-Kuan Wang, Chi-Yuan Li, Kuo-Ting Sun
|
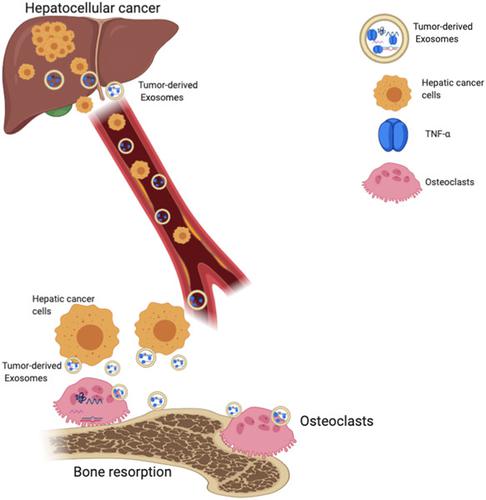
Bone is the common extra-hepatic site for cancer metastasis. Hepatic cancer is associated with a higher incidence of pathological fracture. However, this important regulatory mechanism remains unexplored. Thus, exosome-mediated cell-cell communication between hepatocellular cancer and bone might be key to osteolytic bone destruction. Huh-7 exosomes were characterized for size and exosome marker expressions (CD63, Alix). Exosome mediated osteoclast differentiation in the RAW 264.7 cells was monitored from day 1 to 6 and multinucleated osteoclast formation and bone resorption activity were analyzed. The osteoclastogenic factor expressions in the exosomes and osteoclast differentiation markers such as tumor necrosis factor receptor 6 (TRAF6), nuclear factor κB (NF-κB), nuclear factor of activated T-cells, cytoplasmic 1 (NFATc1), and cathepsin K (CTSK) were analyzed using western blot. Exosomes released by liver cancer cells (Huh-7) promoted osteoclast differentiation in RAW 264.7 cells. Analysis of osteoclastogenic factors in the exosomes showed that exosomes were specifically enriched with tumor necrosis factor α (TNF-α). Huh-7 exosomes promoted osteoclast differentiation by significantly increasing the number of TRAP-positive multi nucleated osteoclasts and resorption pits. Importantly, exosomes upregulated osteoclast markers TRAF6, NF-κB, and CTSK expressions. Further, neutralizing exosomal TNF-α reverted exosome-mediated osteoclast differentiation in RAW 264.7 cells. Collectively, our findings show that cellular communication of exosomal TNF-α from hepatocellular cancer cells (Huh-7) regulates osteoclast differentiation through NF-κB/CTSK/TRAP expressions. Thus, exosomal TNF-α might act as an important therapeutic target to prevent hepatocellular cancer mediated pathological bone disease.
中文翻译:

来自肝细胞癌细胞的外泌体肿瘤坏死因子-α (Huh-7) 促进破骨细胞分化
骨是癌症转移的常见肝外部位。肝癌与较高的病理性骨折发生率相关。然而,这一重要的调节机制仍未被探索。因此,外泌体介导的肝细胞癌和骨之间的细胞间通讯可能是溶骨性骨破坏的关键。Huh-7 外泌体的大小和外泌体标记表达(CD63,Alix)进行了表征。从第 1 天到第 6 天监测 RAW 264.7 细胞中外泌体介导的破骨细胞分化,并分析多核破骨细胞的形成和骨吸收活性。外泌体中破骨细胞因子的表达和破骨细胞分化标志物如肿瘤坏死因子受体 6 (TRAF6)、核因子 κB (NF-κB)、活化 T 细胞核因子、细胞质 1 (NFATc1)、使用蛋白质印迹分析组织蛋白酶 K (CTSK)。肝癌细胞 (Huh-7) 释放的外泌体促进 RAW 264.7 细胞中的破骨细胞分化。外泌体中破骨细胞因子的分析表明,外泌体特异性富集肿瘤坏死因子α(TNF-α)。Huh-7外泌体通过显着增加TRAP阳性多核破骨细胞和吸收坑的数量来促进破骨细胞分化。重要的是,外泌体上调破骨细胞标志物 TRAF6、NF-κB 和 CTSK 的表达。此外,中和外泌体 TNF-α 可逆转 RAW 264.7 细胞中外泌体介导的破骨细胞分化。总的来说,我们的研究结果表明,来自肝细胞癌细胞 (Huh-7) 的外泌体 TNF-α 的细胞通讯通过 NF-κB/CTSK/TRAP 表达调节破骨细胞分化。因此,
更新日期:2021-08-12
中文翻译:

来自肝细胞癌细胞的外泌体肿瘤坏死因子-α (Huh-7) 促进破骨细胞分化
骨是癌症转移的常见肝外部位。肝癌与较高的病理性骨折发生率相关。然而,这一重要的调节机制仍未被探索。因此,外泌体介导的肝细胞癌和骨之间的细胞间通讯可能是溶骨性骨破坏的关键。Huh-7 外泌体的大小和外泌体标记表达(CD63,Alix)进行了表征。从第 1 天到第 6 天监测 RAW 264.7 细胞中外泌体介导的破骨细胞分化,并分析多核破骨细胞的形成和骨吸收活性。外泌体中破骨细胞因子的表达和破骨细胞分化标志物如肿瘤坏死因子受体 6 (TRAF6)、核因子 κB (NF-κB)、活化 T 细胞核因子、细胞质 1 (NFATc1)、使用蛋白质印迹分析组织蛋白酶 K (CTSK)。肝癌细胞 (Huh-7) 释放的外泌体促进 RAW 264.7 细胞中的破骨细胞分化。外泌体中破骨细胞因子的分析表明,外泌体特异性富集肿瘤坏死因子α(TNF-α)。Huh-7外泌体通过显着增加TRAP阳性多核破骨细胞和吸收坑的数量来促进破骨细胞分化。重要的是,外泌体上调破骨细胞标志物 TRAF6、NF-κB 和 CTSK 的表达。此外,中和外泌体 TNF-α 可逆转 RAW 264.7 细胞中外泌体介导的破骨细胞分化。总的来说,我们的研究结果表明,来自肝细胞癌细胞 (Huh-7) 的外泌体 TNF-α 的细胞通讯通过 NF-κB/CTSK/TRAP 表达调节破骨细胞分化。因此,


























 京公网安备 11010802027423号
京公网安备 11010802027423号