Bioorganic & Medicinal Chemistry ( IF 3.5 ) Pub Date : 2021-08-12 , DOI: 10.1016/j.bmc.2021.116344 Haiyan Yao 1 , Quanping Guo 1 , Mengran Wang 1 , Rui Wang 2 , Zhaoqing Xu 2
|
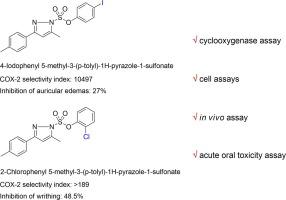
Based on a new pyrazole sulfonate synthetic method, a novel class of molecules with a basic structure of pyrazole N-aryl sulfonate have been designed and synthesized. The interest in conducting intensive research stems from quite evident anti-inflammatory effects exhibited by the compounds in preliminary animal experiments. A series of compounds were synthesized by different substitutions of the R1, R2, and R3 groups. Within the series, 4-iodophenyl 5-methyl-3-(p-tolyl)-1H-pyrazole-1-sulfonate and phenyl 5-methyl-3-(4-(trifluoromethyl) phenyl)-1H-pyrazole-1-sulfonate exhibited excellent anti-inflammatory activity (% inhibition of auricular edemas = 27.0 and 35.9, respectively); the in vivo analgesic activity of phenyl 5-methyl-3-(p-tolyl)-1H-pyrazole-1-sulfonate and 2-chlorophenyl 5-methyl-3-(p-tolyl)-1H-pyrazole-1-sulfonate was confirmed to be effective (inhibition ratio of writhing = 50.7% and 48.5% separately), and compounds phenyl 5-methyl-3-(p-tolyl)-1H-pyrazole-1-sulfonate , 4-iodophenyl 5-methyl-3-(p-tolyl)-1H-pyrazole-1-sulfonate and 2-chlorophenyl 5-methyl-3-(p-tolyl)-1H-pyrazole-1-sulfonate were identified as selective COX-2 inhibitors (SI = 455, 10,497 and >189 severally). In Acute Oral Toxicity assays conducted in vivo, the lethal dose 50 (LD50) of 4-iodophenyl 5-methyl-3-(p-tolyl)-1H-pyrazole-1-sulfonate and 2-chlorophenyl 5-methyl-3-(p-tolyl)-1H-pyrazole-1-sulfonate to mice was >2000 mg/kg BW.
中文翻译:

吡唑N-芳基磺酸盐的发现:一种新型高效的环氧合酶-2 (COX-2) 选择性抑制剂
基于一种新的吡唑磺酸盐合成方法,设计并合成了一类具有吡唑N-芳基磺酸盐基本结构的新型分子。进行深入研究的兴趣源于这些化合物在初步动物实验中表现出的相当明显的抗炎作用。通过R 1、R 2和R 3基团的不同取代,合成了一系列化合物。在该系列中,4-iodophenyl 5-methyl-3-(p-tolyl)-1H-pyrazole-1-sulfonate 和 phenyl 5-methyl-3-(4-(trifluoromethyl) phenyl)-1H-pyrazole-1-sulfonate表现出优异的抗炎活性(耳廓水肿抑制百分比分别为 27.0 和 35.9);在体内5-甲基-3-(p-tolyl)-1H-pyrazole-1-sulfonate 和 2-chlorophenyl 5-methyl-3-(p-tolyl)-1H-pyrazole-1-sulfonate 的镇痛活性被证实为有效(扭体抑制率分别为50.7%和48.5%),化合物苯基5-甲基-3-(对甲苯基)-1H-吡唑-1-磺酸盐、4-碘苯基5-甲基-3-(对-甲苯基) tolyl)-1H-pyrazole-1-sulfonate 和 2-chlorophenyl 5-methyl-3-(p-tolyl)-1H-pyrazole-1-sulfonate 被鉴定为选择性 COX-2 抑制剂(SI = 455、10,497 和 >189几个)。在体内进行的急性口服毒性试验中,4-碘苯基 5-甲基-3-(对甲苯基)-1H-吡唑-1-磺酸盐和 2-氯苯基 5-甲基-3-的致死剂量为 50 (LD 50 ) (p-tolyl)-1H-pyrazole-1-sulfonate 对小鼠的影响 >2000 mg/kg BW。


























 京公网安备 11010802027423号
京公网安备 11010802027423号