当前位置:
X-MOL 学术
›
Chem. Soc. Rev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Carbonyl umpolung as an organometallic reagent surrogate
Chemical Society Reviews ( IF 46.2 ) Pub Date : 2021-08-12 , DOI: 10.1039/d1cs00418b Xi-Jie Dai 1 , Chen-Chen Li 1 , Chao-Jun Li 1
Chemical Society Reviews ( IF 46.2 ) Pub Date : 2021-08-12 , DOI: 10.1039/d1cs00418b Xi-Jie Dai 1 , Chen-Chen Li 1 , Chao-Jun Li 1
Affiliation
|
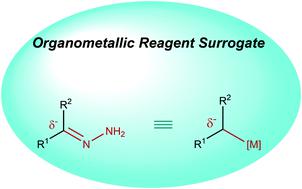
Construction of new carbon–carbon bonds is the cornerstone of organic chemistry. Organometallic reagents are amongst the most robust and versatile nucleophiles for this purpose. Polarization of the metal–carbon bonds in these reagents facilitates their reactions with a vast array of electrophiles to achieve chemical diversification. The dependence on stoichiometric quantities of metals and often organic halides as feedstock precursors, which in turn produces copious amounts of metal halide waste, is the key limitation of the classical organometallic reactions. Inspired by the classical Wolff–Kishner reduction converting carbonyl groups in aldehydes or ketones into methylene derivatives, our group has recently developed strategies to couple various alcohols, aldehydes, and ketones with a broad range of both hard and soft carbon electrophiles in the presence of catalytic amounts of transition metals, via the hydrazone derivatives: i.e., as organometallic reagent surrogates. This Tutorial Review describes the chronological development of this concept in our research group, detailing its creation in the context of a deoxygenation reaction and evolution to a more general carbon–carbon bond-forming strategy. The latter is demonstrated by the employment of carbonyl-derived alkyl carbanions in various transition-metal catalyzed chemical transformations, including 1,2-carbonyl/imine addition, conjugate addition, carboxylation, olefination, cross-coupling, allylation, alkylation and hydroalkylation.
中文翻译:

羰基离子化作为有机金属试剂替代物
构建新的碳-碳键是有机化学的基石。为此目的,有机金属试剂是最稳定和通用的亲核试剂之一。这些试剂中金属-碳键的极化促进了它们与大量亲电子试剂的反应,以实现化学多样化。依赖化学计量的金属和通常有机卤化物作为原料前体,这反过来产生大量金属卤化物废物,是经典有机金属反应的关键限制。受经典 Wolff-Kishner 还原将醛或酮中的羰基转化为亚甲基衍生物的启发,我们小组最近开发了将各种醇、醛、通过腙衍生物:即作为有机金属试剂的替代物。本教程回顾描述了我们研究小组中这个概念的时间顺序发展,详细介绍了它在脱氧反应和演变为更一般的碳-碳键形成策略的背景下的创建。后者通过在各种过渡金属催化的化学转化中使用羰基衍生的烷基碳负离子来证明,包括 1,2-羰基/亚胺加成、共轭加成、羧化、烯化、交叉偶联、烯丙基化、烷基化和加氢烷基化。
更新日期:2021-08-12
中文翻译:

羰基离子化作为有机金属试剂替代物
构建新的碳-碳键是有机化学的基石。为此目的,有机金属试剂是最稳定和通用的亲核试剂之一。这些试剂中金属-碳键的极化促进了它们与大量亲电子试剂的反应,以实现化学多样化。依赖化学计量的金属和通常有机卤化物作为原料前体,这反过来产生大量金属卤化物废物,是经典有机金属反应的关键限制。受经典 Wolff-Kishner 还原将醛或酮中的羰基转化为亚甲基衍生物的启发,我们小组最近开发了将各种醇、醛、通过腙衍生物:即作为有机金属试剂的替代物。本教程回顾描述了我们研究小组中这个概念的时间顺序发展,详细介绍了它在脱氧反应和演变为更一般的碳-碳键形成策略的背景下的创建。后者通过在各种过渡金属催化的化学转化中使用羰基衍生的烷基碳负离子来证明,包括 1,2-羰基/亚胺加成、共轭加成、羧化、烯化、交叉偶联、烯丙基化、烷基化和加氢烷基化。



























 京公网安备 11010802027423号
京公网安备 11010802027423号