Journal of Organometallic Chemistry ( IF 2.3 ) Pub Date : 2021-08-11 , DOI: 10.1016/j.jorganchem.2021.122024 Fang Yong 1 , Junxia Yang 1 , Zhaomin Wei 2 , Lei Zhang 1 , Wenli Yuan 1 , Guohong Tao 1 , Song Qin 1
|
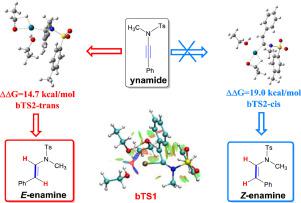
The reaction mechanism of the Pd(PPh3)4-catalyzed stereo-selective hydrogenation of ynamide to enamine with ethanol as a hydrogenation agent was investigated using density functional theory (DFT). The computational study provides the unique multistep hydrogenation pathway with the reasonable energy barrier, and the low energy barriers through the proton shuttle process are well rationalized. The calculation predicts that the ynamide undergoes stepwise hydrogenation by Pd-activated ethanol species, Pd(PPh3)4 acts as the precursor and the (EtOH)2-Pd-ynamide complex serves as active catalyst in the reaction. The calculation on the ynamide system indicates that there exist six competing reaction paths. For each path, the overall energy barrier is identified as the step corresponding to the oxidative addition of the O‒H bond of the hydrogenating agent to palladium center of the catalytic-complex. Ethanol could play as a proton shuttle in the hydrogen transfer step and therefore dramatically decreases the energy barrier for this concerned transition state, which is further validated by turnover frequency (TOF) model. The high selectivity towards enamine is attributed to the post-transition-state dynamics in the second hydrogenation stage, which leads exclusively to the dissociation of the product. The predominant product with E-configuration is reproduced theoretically, which is consistent with the experimental observation. Reduced density gradient (RDG) analysis and atoms in molecules (AIM) of the transition states confirm the significant influence of ethanol on the mechanism and stereo selectivity.
中文翻译:

炔酰胺的催化立体选择性氢化得到烯胺:乙醇作为氢供体
使用密度泛函理论(DFT)研究了以乙醇为氢化剂的Pd(PPh 3 ) 4催化的炔酰胺立体选择性氢化成烯胺的反应机理。计算研究为独特的多步氢化途径提供了合理的能垒,通过质子穿梭过程的低能垒得到了很好的合理化。计算预测,ynamide 被 Pd 活化的乙醇物质逐步加氢,Pd(PPh 3 ) 4作为前体,(EtOH) 2-Pd-炔酰胺配合物在反应中作为活性催化剂。对ynamide 系统的计算表明存在六个竞争反应路径。对于每条路径,总能垒被确定为对应于氢化剂的 O-H 键氧化加成到催化复合物的钯中心的步骤。乙醇可以在氢转移步骤中充当质子穿梭机,因此显着降低了这种相关过渡态的能量势垒,周转频率 (TOF) 模型进一步验证了这一点。对烯胺的高选择性归因于第二氢化阶段的过渡态后动力学,这仅导致产物的离解。带E的主要产品-配置在理论上重现,这与实验观察一致。降低的密度梯度 (RDG) 分析和过渡态分子中的原子 (AIM) 证实了乙醇对机理和立体选择性的显着影响。


























 京公网安备 11010802027423号
京公网安备 11010802027423号