Materials Today Energy ( IF 9.3 ) Pub Date : 2021-08-11 , DOI: 10.1016/j.mtener.2021.100831 Kai S. Exner 1, 2, 3
|
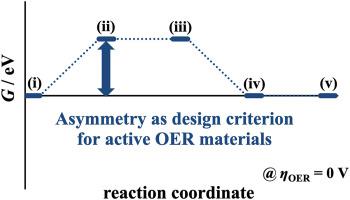
The development of oxygen evolution reaction (OER) electrocatalysts has been spurred by thermodynamic considerations on the free-energy landscape. It is a common paradigm that the optimum thermodynamic free-energy landscape reveals a symmetric shape in that all reaction intermediates are stabilized at the equilibrium potential of the reaction. However, so far, no OER electrocatalyst has been reported that corresponds to the thermodynamic ideal because of the presence of a linear scaling relationship. Therefore, the common approach builds on the breaking of the scaling relations to establish a catalytic material that is close to the symmetric picture, yet, with minor successes. Relating to the simple two-electron hydrogen evolution reaction (HER), it was recently reported that the optimum thermodynamic free-energy landscape reveals an asymmetric shape rather than a symmetric form as soon as overpotential and kinetic effects are factored in the analysis. This finding motivates scrutinizing whether the symmetric free-energy landscape as the thermodynamic ideal in the OER is justified. Transferring the knowledge from the HER to the OER results in the introduction of the electrochemical-step asymmetry index (ESAI), representing the concept of the asymmetric thermodynamic free-energy diagram. By comparing the ESAI to the symmetric picture in terms of the electrochemical-step symmetry index (ESSI), it is demonstrated herein that the asymmetric rather than the symmetric free-energy landscape corresponds to the thermodynamic ideal. This outcome suggests changing the mindset when applying the concept of free-energy diagrams for the discovery of OER materials by heuristic material-screening techniques.
中文翻译:

为什么析氧反应的最佳热力学自由能图显示不对称形状
析氧反应 (OER) 电催化剂的发展受到对自由能景观的热力学考虑的推动。最佳热力学自由能图显示对称形状是一个常见的范例,因为所有反应中间体都稳定在反应的平衡电位。然而,到目前为止,由于存在线性标度关系,还没有报道符合热力学理想的 OER 电催化剂。因此,常见的方法建立在打破比例关系的基础上,以建立一种接近对称图的催化材料,但取得了较小的成功。关于简单的两电子析氢反应(HER),最近有报道称,一旦在分析中考虑了过电位和动力学效应,最佳热力学自由能景观就会显示出不对称的形状而不是对称的形式。这一发现激发了仔细审查作为 OER 中热力学理想的对称自由能景观是否合理。将知识从 HER 转移到 OER 导致引入了电化学阶梯不对称指数 (ESAI),代表了不对称热力学自由能图的概念。通过将 ESAI 与电化学阶梯对称指数 (ESSI) 方面的对称图片进行比较,本文证明不对称而不是对称的自由能景观对应于热力学理想。



























 京公网安备 11010802027423号
京公网安备 11010802027423号