当前位置:
X-MOL 学术
›
Tetrahedron
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Azomethine ylide cycloaddition of 2-C-formyl glycals with α-amino acids for the synthesis of substituted pyrroles
Tetrahedron ( IF 2.1 ) Pub Date : 2021-08-10 , DOI: 10.1016/j.tet.2021.132389 V. Veerabadhra Reddy 1 , B.V. Subba Reddy 1
中文翻译:

用于合成取代吡咯的 2-C-甲酰基聚糖与 α-氨基酸的偶氮甲碱叶立德环加成反应
更新日期:2021-09-12
Tetrahedron ( IF 2.1 ) Pub Date : 2021-08-10 , DOI: 10.1016/j.tet.2021.132389 V. Veerabadhra Reddy 1 , B.V. Subba Reddy 1
Affiliation
|
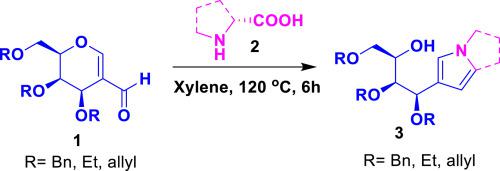
A novel strategy has been devised for the synthesis of pyrrole based acyclo-C-nucleosides, in particular an open-chain sugar substituted pyrrole derivatives by means of the condensation of 2-C-formyl glycals with α-amino acids through an intramolecular azomethine cycloaddition under thermal conditions. The use of cyclic α-amino acids provides the corresponding bicyclic pyrrole derivatives. This is a first report on the synthesis of pyrrole based acyclo-C-nucleosides. 2009 Elsevier Ltd. All rights reserved.
中文翻译:

用于合成取代吡咯的 2-C-甲酰基聚糖与 α-氨基酸的偶氮甲碱叶立德环加成反应
一种新的策略已被设计用于吡咯的基于acyclo-合成Ç核苷,特别是开链糖通过2-缩合来取代的吡咯衍生物Ç甲酰基烯糖用α-氨基酸通过分子内甲亚胺环加成在热条件下。环状α-氨基酸的使用提供了相应的双环吡咯衍生物。这是基于吡咯acyclo-合成的第一报告Ç核苷。2009 Elsevier Ltd. 保留所有权利。



























 京公网安备 11010802027423号
京公网安备 11010802027423号