Tetrahedron ( IF 2.1 ) Pub Date : 2021-08-10 , DOI: 10.1016/j.tet.2021.132381 Ryuichi Murata 1 , Keisuke Asano 1 , Seijiro Matsubara 1
|
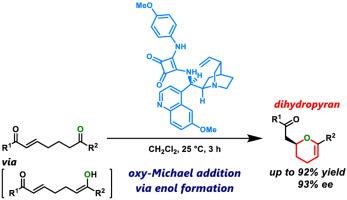
Carbonyl compounds employed as carbon nucleophiles have played a dominant role in synthetic organic chemistry; however, there is very limited use of these compounds as oxygen nucleophiles. In particular, there are only a few reports on the oxy-Michael addition of the enol forms of carbonyl nucleophiles. In this study, we present the asymmetric cycloetherification of enols, which are generated in situ from enone-bearing ketones, using chiral bifunctional organocatalysts bearing amino and squaramide groups. This transformation chemo- and enantioselectively afforded dihydropyran derivatives, which are the core structures of building blocks for synthesizing glycans.
中文翻译:

通过烯醇的分子内氧-迈克尔加成催化不对称环醚化
用作碳亲核试剂的羰基化合物在合成有机化学中发挥了主导作用。然而,这些化合物作为氧亲核试剂的用途非常有限。特别是,关于羰基亲核试剂的烯醇形式的氧-迈克尔加成的报道很少。在这项研究中,我们使用带有氨基和方酸酰胺基团的手性双功能有机催化剂,介绍了烯醇的不对称环醚化,这些烯醇是由带有烯酮的酮原位生成的。这种转化化学和对映选择性地提供了二氢吡喃衍生物,这是合成聚糖的核心结构。



























 京公网安备 11010802027423号
京公网安备 11010802027423号